SBIC ECR Symposium & Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Anti-trypanosomatid activity of [M(L)(PPh3)] (M = Pd, Pt) complexes with L = coumarin-thiosemicarbazone derivatives: inclusion of a nitro group (#526)
In the search of new anti-trypanosomatid drugs, our group has been working on the design of coumarin-thiosemicarbazone hybrids. Different structural variations in the hybrid structure could improve their activity. Additionally, the coordination of potentially anti-trypanosomatid coumarin-thiosemicarbazone hybrids to metals (e.g. Pd(II) or Pt(II)) could also improve the anti-trypanosomal activity of the free ligands and also lower their nonspecific toxicity. Based on the above, we previously reported a family of compounds with the formula [M(L1-L4)(PPh3)] that showed low activity vs. Trypanosoma cruzi, the etiological agent of Chagas´ disease [1].
In this work, we report the synthesis and characterization of a new family of coumarin-thiosemicarbazones including a NO2 group in the coumarin moiety (H2L5-H2L8). This group was selected because it is present in the pharmacophore of Nifurtimox, a clinically approved drug for Chagas´s disease and human African trypanosomiasis. In addition, we present the preparation of two new families of complexes with the formula [M(L5-L8)(PPh3)], M = Pd, Pt. The nitro-containing ligands and their metal complexes showed an improved anti-T. cruzi activity. Furthermore, we tested both families of ligands H2L1-H2L4 and H2L1-H2L4 as well as [M(L1-L4)(PPh3)] complexes on T. brucei brucei parasite (African trypanosoma). Interestingly, the activities and selectivities were significantly higher than those vs. T. cruzi (e.g. sub-micromolar IC50 and SI up to 230, calculated as SI = IC50 macrophages / IC50 T. brucei). Further studies are in progress to determine the activity of [M(L5-L8)(PPh3)] vs. T. b. b. and to explain the differences between the observed activities in such closely related parasites.
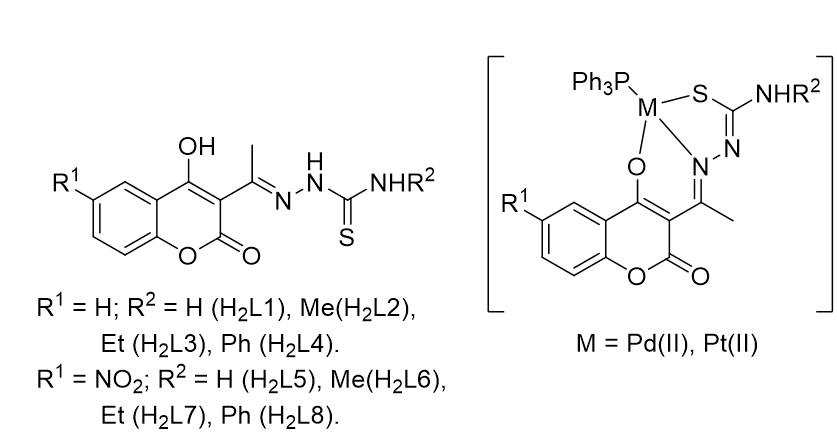
Figure 1. Left: prepared coumarin-thiosemicarbazone hybrids. Right: generic structure of the obtained complexes.
- [1] Rostán, S., Pozo-Martínez, J., Arcos, M. A., Moncada-Basualto, M., Aguilera, E., Alvarez, N., ... & Otero, L. (2024). Pt (II) and Pd (II) complexes with coumarin-thiosemicarbazone hybrid ligands and triphenylphosphine coligand as potential anti T. cruzi agents. Journal of Molecular Structure, 1313, 138711.