SBIC ECR Symposium & Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Pt(II) organometallic complexes for the therapy of viral and parasitic tropical neglected diseases infections (#459)
Our research group has been investigating metal-based compounds for the treatment of neglected diseases, such as arboviruses and leishmaniasis.1 In this work, Pt(II) NˆC phenylpyridine compounds were modified with phosphines generating the structures.(Figure 1) Among them 5 is a novel structure, characterized by spectroscopic techniques. The compounds are stable in DMSO and showed negligible chloride exchange reaction with N-acetyl-L-cysteine, demonstrating its stability with the thiol group. The partition coefficient (Log P) ranges 0.23 to 1.25, demonstrating their general lipophilicity. Complex 2 is the most lipophilic, 3 presents in the middle of the range and 5 is the most hydrophilic. Except by 5, the compounds presented interesting inhibition of Zika or Mayaro viruses (75 – 95 %) at non-cytotoxic concentrations, which is high (50 µM), as all the compounds are not cytotoxic to mammalian cells. Complex 3, seem to present the best lipophilic/hydrophilic balance of the series, and it was the only one to show activity against Leishmania (L.) amazonensis promastigotes. The EC50 of 3 is 3 μM and SI = 10. When evaluated the reduction of infection rates the compound presented 25% reduction. Interactions with BSA showed both dynamic and static quenching and a binding constant of 104 M-1, while the spectrophotometry of complexes interactions with DNA revealed hyperchromic or hypochromic effects depending on the complex, suggesting 3 and 4 can intercalate DNA. Except by 5, the complexes do not present intrinsic fluoresce in DMSO. Among the complexes, it was highlighted that Complex 3 exhibited fluorescence after interacting with histidine at Tris HCl pH 7.4 and acetate buffer pH 5.2. 2 (Figure 2) Both the free and conjugated complexes exhibited significant fluorescence.
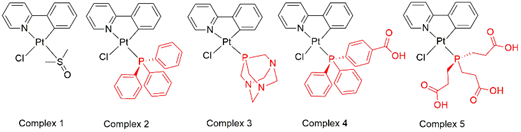
Figure 1 : Structure of the five platinum complexes
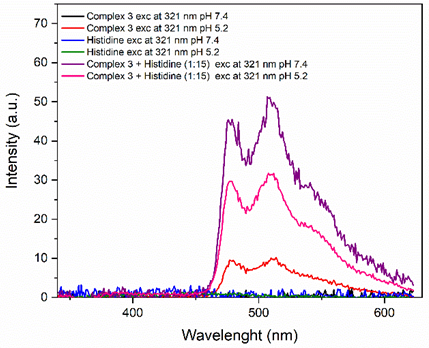
Figure 2: Emission spectrum of complex 2, Histidine
and complex 2 + Histidine in the ratio 1:15 at pH 7,4 and 5,2.
- L. S. Oliveira et al., ChemBioChem, 25, 6, 1–11, 2024
- A. I. Solomatina et al., Bioconjug. Chem., 28, 2, 426–437, 2017.