SBIC ECR Symposium & Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Decoding factors lead to reaction versatility in non-heme iron and 2-oxoglutarate dependent enzymes (#455)
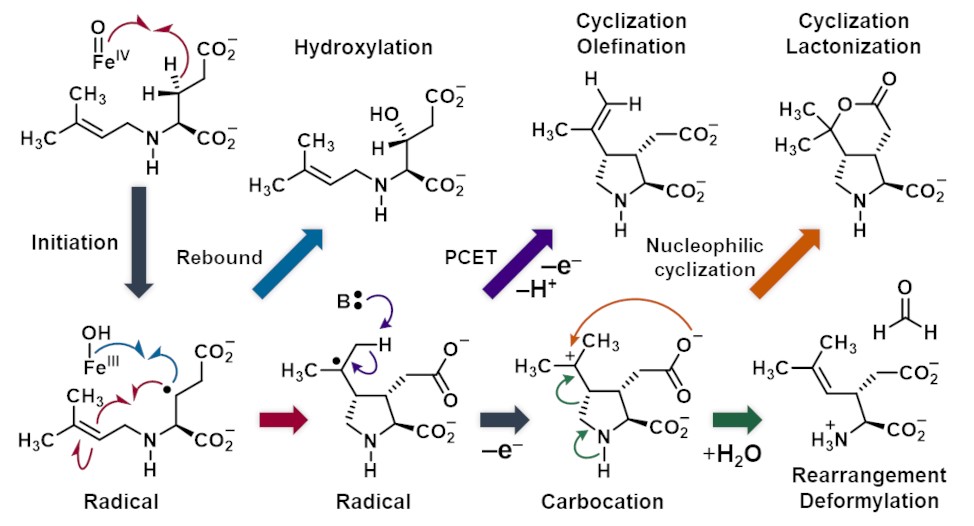
Non-heme iron and 2-oxoglutarate dependent (Fe/2OG) enzymes catalyze synthetic challenging reactions with precise control through molecular recognition, where a ferrous cofactor is employed to activate molecular oxygen with an aid of 2OG, forming a reactive iron(IV)–oxo (ferryl) species. This key intermediate enables C–H bond activation, leading to Fe(III)–OH and a substrate radical. Besides C–OH bond formation (hydroxylation) that proceeds through oxygen rebound pathway, Fe/2OG enzymes also catalyze alternative reactions including desaturation, ring formation, etc. In this study, the reaction mechanism of KabC and DabC in the biosynthesis of neuroexcitatory kainoids are fully mapped. Through a combination of biochemical assays with the substrate N-dimethylallyl L-glutamate isotopologs and radical clock analogs, stopped-flow and other biophysical experiments, and calculation, we demonstrate multiple branching takes place including both radical and carbocationic species. This results in a combination of radical-mediated hydroxylation, radical-mediated C–C bond formation, intramolecular nucleophilic addition, desaturation likely mediated by PCET, and C–C bond cleavage via a retro-aza-Prins reaction. Collectively, these observations reveal the complexity of Fe/2OG enzymes-catalyzed reactions, providing insights for future protein engineering efforts aimed at designing Fe/2OG enzymes with tailored catalytic properties for synthetic biology and pharmaceutical applications.