SBIC ECR Symposium & Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Cross-linking metal binding sites on proteins using dimetallic compounds (#428)
Cancer is a leading cause of death globally, often characterised by the dysregulation of proteins. Proteins are the principal choice in targeted drug development,1 especially for metal-based drugs that can form covalent bonds to amino acid side-chains.1 Formation of these drug–protein adducts is integral to the transport, uptake, and cytotoxicity of many metal-based anticancer drug candidates.2 A notable example of this is BOLD-100, currently in Phase III of clinical trials, that metallates the transport protein human serum albumin, and imparts a cytotoxic effect once in the cells.3 However, the hunt for greater specificity towards target sites on proteins is ongoing, and thus we present a novel strategy to increase the binding specificity towards proteins. We aim to use dimetallic compounds to cross-link amino acid binding sites, which chelate the protein through the architecture of the metallocompound, generating avidity.
The interactions of rationally-designed homobimetallic compounds (1a–1d) with the model protein hen egg white lysozyme (HEWL), characterised by protein crystallographic (Fig. 1) and top-down mass spectrometric methods, will be discussed. The in vitro cytotoxicity of the compounds in cancer cell lines will also be presented. This work builds upon the plethora of monometallic compounds bound to proteins by the inclusion of a second metal centre, greatly enhancing the complexity of the system, offering potential as more effective protein-targeting compounds.
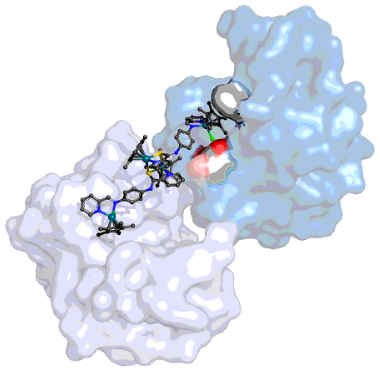
Figure 1. The interaction of a dimeric Rh compound with D101 and W62 of HEWL (marine blue), and a symmetry-related HEWL molecule (light blue).
- Wilson, A. J. Inhibition of protein-protein interactions using designed molecules. Chem. Soc. Rev. 2009, 38 (12), 3289-3300.
- Simovic, A. R.; Masnikosa, R.; Bratsos, I.; Alessio, E. Chemistry and reactivity of ruthenium(II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord. Chem. Rev. 2019, 398, 1103011.
- Bijelic, A.; Theiner, S.; Keppler, B. K.; Rompel, A. X-ray Structure Analysis of Indazolium trans-[Tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019) Bound to Human Serum Albumin Reveals Two Ruthenium Binding Sites and Provides Insights into the Drug Binding Mechanism. J. Med. Chem. 2016, 59 (12), 5894-5903.