Oral Presentation 21st International Conference on Biological Inorganic Chemistry 2025
The Specificity of the Plectin–Plecstatin-1 Interaction (#429)
Increasingly, there is a trend toward anticancer chemotherapeutics that target a single protein. However, the current generation of metal-based anticancer drugs lack this specificity, limiting their usage.1 To advance novel metal-based anticancer agents toward clinical trials, it is imperative to elucidate the target and mode of action. The novel cytotoxic agent, plecstatin-1 [Ru(η6-p-cymene)(N-(4-fluorophenyl)-2-pyridinecarbothioamide)Cl] has been shown through a proteomics-based target-response profiling approach to have high selectivity for plectin.2 Plectin is a large multi-domain protein (500 kDa, 4,684 amino acids) with two globular ends and a central coiled-coil region. Integral to the cellular cytoskeleton, plectin is a cytolinker connecting cytoskeletal elements such as actin, microtubules, and integrin to uphold cellular structure and regulate cytoskeletal dynamics. Plectin regulates malignant phenotypes that primarily drive metastatic potential, particularly cell migration. Treatment with plecstatin-1 has demonstrated the ability to reorganize the cytoskeletal network and reduce metastatic potential.2 Leveraging the plectin–plecstatin-1 interaction can inhibit the protein–protein interaction between plectin and cytoskeletal components. To fully realize the potential of this interaction, we need to understand the molecular basis of the plectin-plecstatin-1 interaction.
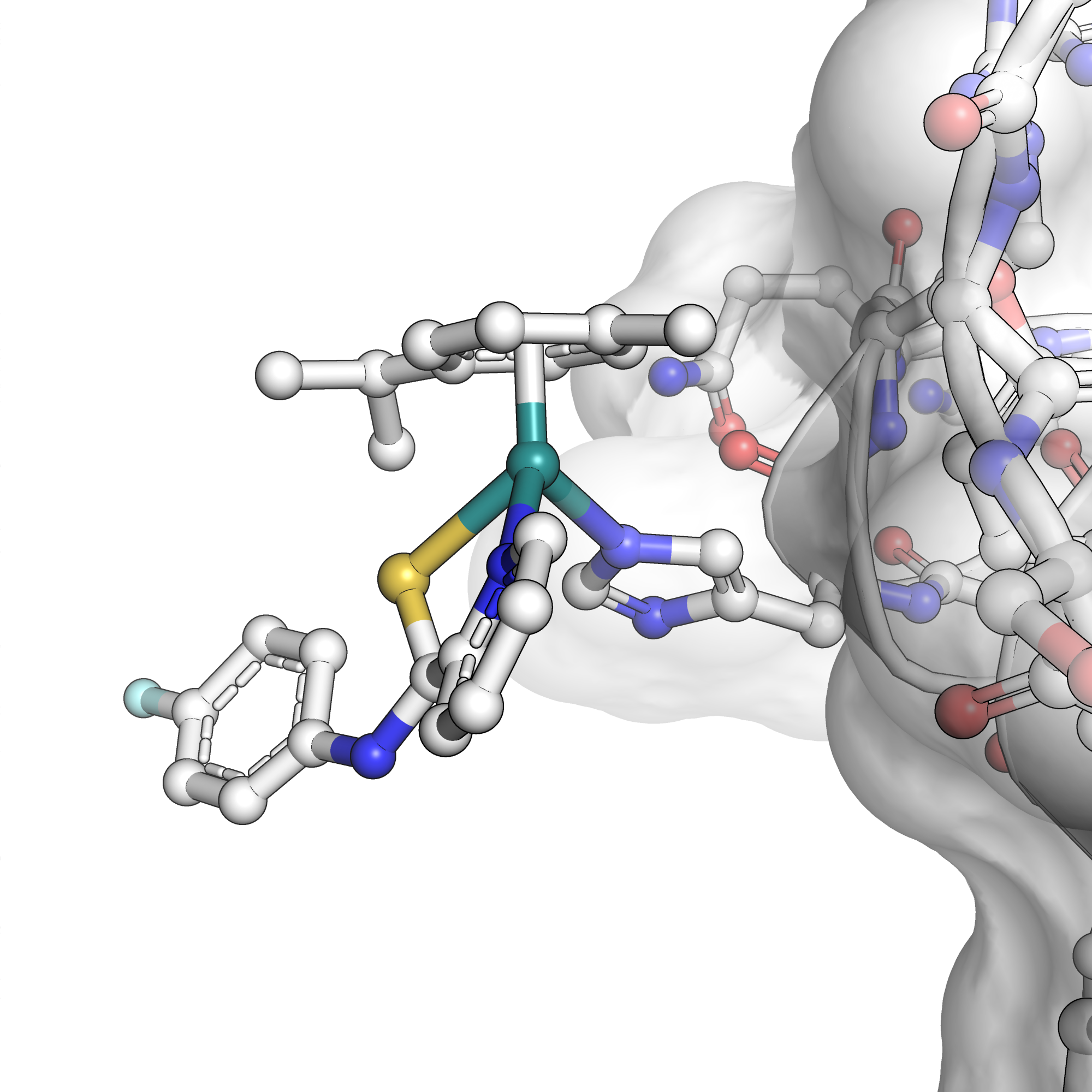
The domains of plectin were recombinantly expressed, forming overlapping constructs. The individual constructs were incubated with plecstatin-1, separated by gel electrophoresis, and imaged using laser ablation inductively coupled plasma mass spectrometry. In an alternative approach, surface plasmon resonance was used to study the interaction directly. These techniques showed that plecstatin-1 favourably interacted with the actin-binding domain of plectin. A crystal structure of the actin-binding domain when incubated with plecstatin-1 was obtained (Fig. 1). Site-directed mutagenesis was used to probe the direct plecstatin-1 binding site on the protein. These studies represent essential steps toward advancing plecstatin-1 towards clinical trials.
References
(1) Von Hoff, D. D.; Schilsky, R.; Reichert, C. M.; Reddick, R. L.; Rozencweig, M.; Young, R. C.; Muggia, F. M. Cancer Treat. Rep. 1979, 63 (9-10), 1527-1531.
(2) Meier, S. M.; Kreutz, D.; Winter, L.; Klose, M. H. M.; Cseh, K.; Weiss, T.; Bileck, A.; Alte, B.; Mader, J. C.; Jana, S.; et al. Angew. Chem., Int. Ed. Engl. 2017, 56 (28), 8267-8271.