Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Inspired by nature: Structural and functional models for vanadium-dependent haloperoxidases (#604)
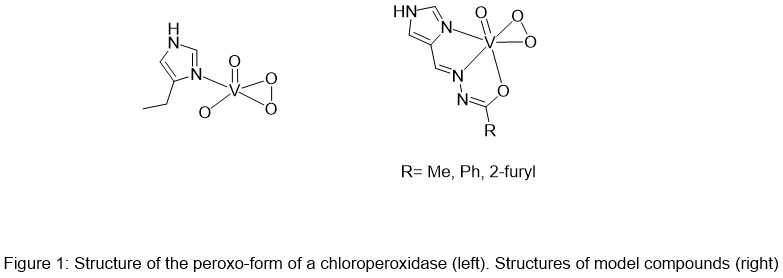
Halogenation of organic compounds is an important process in nature. A diverse structural variety of halogenated species are produced by many marine organisms including algae and kelp. Such biological halogenation reactions are catalyzed by haloperoxidases, which contain either vanadium or iron in their active centres. The mechanism of halogenation is thought to involve formation of hypohalous acids (HXO) by H2O2-oxidation of halide ions. The active site of the peroxo-from of a vanadium-dependent haloperoxidase contains an oxovanadium(V) center coordinated by a histidine residue, a peroxo group and a further oxygen ligand.
In this work, we present various oxovanadium(V) peroxo-complexes which were studied as haloperoxidase models (Figure 1). The chosen ligands are tridentate monoanionic hydrazones derived from condensation of 2-imidazole carbaldehyde and a hydrazide. In the presence of suitable vanadium precursors and H2O2, dark orange peroxo-complexes can be isolated in good yields. The compounds were fully characterized by various methods including 51V-NMR- and IR-spectroscopy, mass spectrometry and single crystal X-ray diffraction.
The coordination environment of the complexes is very similar to that found in the crystal structure of the enzyme. In some structures, an O-bound solvent molecule (H2O or MeOH) is also present. The haloperoxidase activity of the compounds was studied in model reactions by UV-spectroscopy.
- J.-B. Fournier, C. Leblanc, in Outstanding Marine Molecules (Eds.: S. La Barre, J.-M. Kornprobst), Wiley VCH, 2014.
- D. C. Reis, A. A. R. Despaigne, J. G. Da Silva, N. F. Silva, C. F. Vilela, I. C. Mendes, J. A. Takahashi, H. Beraldo, Molecules 2013, 18, 12645.
- G. J. Colpas, B. J. Hamstra, J. W. Kampf, V. L. Pecoraro, J. Am. Chem. Soc. 1996, 118, 3469.
- A. Butler, Curr. Opp. Chem. Biol. 1998, 2, 279.