Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Cationic ligand modifications of iron catalysts enable hydrazine-selective N2 fixation (#607)
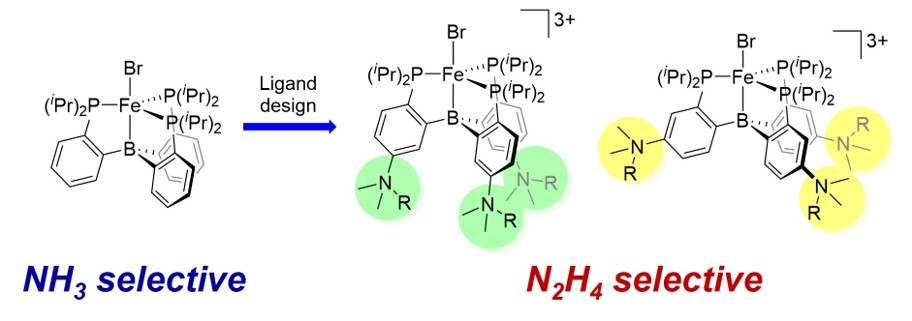
Catalytic nitrogen reduction reaction (N2RR) is an important chemical transformation in both biology and industry, enabling the conversion of inert atmospheric N₂ into valuable nitrogen-containing compounds, such as ammonia (NH₃) and hydrazine (N₂H₄). Our group has demonstrated that tris(phosphino)borane-bound iron complexes can act as well-defined platforms for catalytic N₂ fixation to produce ammonia or hydrazine, where the product selectivity can switch by the choice of proton (H⁺) and electron (e⁻) sources.1,2 In this work, we showcase an iron catalyst design strategy that enables the selective N₂-to-N2H4 conversion under protic conditions. The incorporation of cationic trimethylammonium (NMe₃⁺) groups into the tris(phosphino)borane ligand framework produces Fe-precatalysts that operate efficiently in methanol. The newly prepared iron catalysts achieved hydrazine-selective N₂ fixation with high yields (up to >20:1 N2H4:NH3 selectivity, 73% yield). Computational analyses reveal that these ligand modifications significantly shift the reduction potential of Fe–hydrazido intermediates anodically, which is a key branch point for hydrazine versus ammonia selectivity.3
- Chalkley, M. J.; Del Castillo, T. J.; Matson, B. D.; Roddy, J. P.; Peters, J. C. ACS Cent. Sci. 2017, 3, 217–223.
- Boyd, E. A.; Peters, J. C. J. Am. Chem. Soc. 2023, 145, 14784–14792.
- Manuscript in preparation.