Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Tau is a metal binding protein: Insights of the copper and zinc coordination sites and their impact on aggregation. (#580)
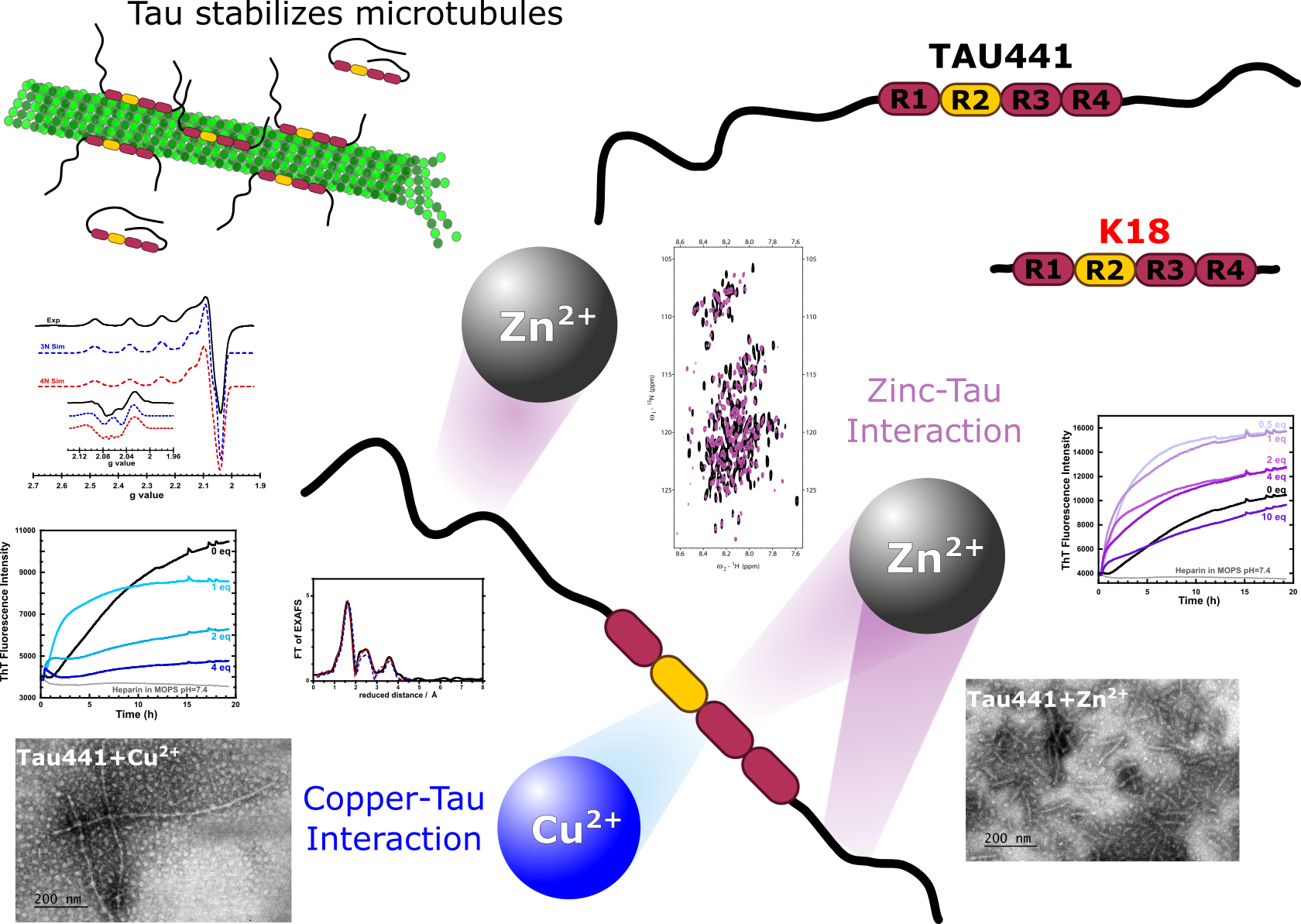
Tau is a protein expressed in the central nervous system; it is located mainly in axons, stabilizing the structure and growth of microtubules. In Alzheimer's disease (AD), Tau undergoes hyperphosphorylation and forms aggregates that give rise to Neurofibrillary Tangles (NFTs). NFTs, together with the presence of senile plaques formed by β-Amyloid peptide (Aβ) and an imbalance of metal ion homeostasis (copper and zinc), are the main hallmarks of AD. Cu and Zn accumulate in AD-related amyloid plaques, and the metal binding properties of Aβ have been extensively studied in the last decades.1 In contrast, only few studies have probed the interaction of metal ions with tau and their presence in NFTs2,3.
In this study, the impact of Cu2+ and Zn2+ ions in the amyloid aggregation of recombinant full-length Tau441 protein was evaluated, finding that these metal ions accelerate protein aggregation and promote oligomerization via disulfide-bridging. Cu2+ and Zn2+ binding to Tau441 protein was probed using electron paramagnetic resonance (EPR), circular dichroism (CD), nuclear magnetic resonance (NMR) and X-ray absorption (XAS) spectroscopies. Tau441 displays only one Cu2+ binding site located in the microtubule binding domain (MTBD), while XAS and NMR results suggest the presence of two Zn2+ binding sites. The metal binding properties of Tau441 were also compared to those of the shorter Tau K18 protein and peptide models for the MTBD, finding that only TauK18 can reproduce the metal binding sited of Tau441, and underscoring the importance of studying the full-length protein. Our results demonstrate that essential metal ions, such as Cu2+ and Zn2+, bind to Tau and impact its amyloid aggregation. The fact that Tau is a metal binding protein opens up new facets of the biological inorganic chemistry of AD.
- Y. Posadas, V.E. López-Guerrero, T. Arcos-López, R.I. Sayler, C. Sánchez-López, J. Segovia, C. Perez-Cruz, L. Quintanar, 2.19 - The role of d-block metal ions in neurodegenerative diseases, in: J. Reedijk, K.R. Poeppelmeier (Eds.), Comprehensive Inorganic Chemistry III (Third Edition), Elsevier, Oxford, 2023, p. 575-628.
- A.Soragni, B. Zambelli, M.D. Mukrasch, J. Biernat, S. Jeganathan, C. Griesinger, S. Ciurli, E. Mandelkow, M. Zweckstetter, Biochemistry 47 (2008) p. 10841-10851.
- L.M. Sayre, G. Perry, P.L.R. Harris, Y. Liu, K.A. Schubert and M.A. Smith. Journal of Neurochemistry 74 (2000) p. 270-279.