Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Tuning metal coordination and ligand affinity in de novo designed metalloproteins via outer-sphere modifications (#520)
Metalloproteins often rely on finely tuned outer coordination sphere interactions to regulate metal reactivity and binding of exogenous ligands.1 Reproducing this level of control in artificial systems remains a major challenge. De novo designed protein scaffolds offer a simplified, modular platform to isolate the specific contributions of these interactions, allowing the identification of individual electrostatic or steric contributions without the complexity of natural metalloenzymes.2 In this work, we present a rationally designed three-stranded coiled coil that binds cobalt(II) through canonical amino acids (Figure 1) and allows precise manipulation of outer-sphere residues. The introduction of positively charged residues (e.g., arginine) near the metal site preserved both metal binding and structural integrity, as shown by X-ray crystallography. Remarkably, electrostatic interactions in the outer coordination sphere reshaped the cobalt(II) coordination geometry, shifted the electronic transitions, and modulated the protonation state of a bound water molecule, elucidated by UV-Vis spectroscopy and X-ray Absorption Spectroscopy (XAS). These effects are strongly dependent on the spatial positioning of the substituted residues, mimicking the finely tuned electrostatic environments found in natural metalloenzyme active sites.3 To further probe the impact of outer coordination sphere interactions, we employed a series of small, negatively charged exogenous ligands, including halides, azide, and thiocyanate, as spectroscopic probes. The observed variations in ligand affinity and coordination number and geometry reinforce the critical role of electrostatics in governing metal–ligand interactions within these artificial systems. These findings demonstrate that the outer coordination sphere can be a powerful design element for tuning the properties of metal centers in artificial protein scaffolds, offering insights for the rational development of functional metalloproteins and metalloenzyme mimetics.
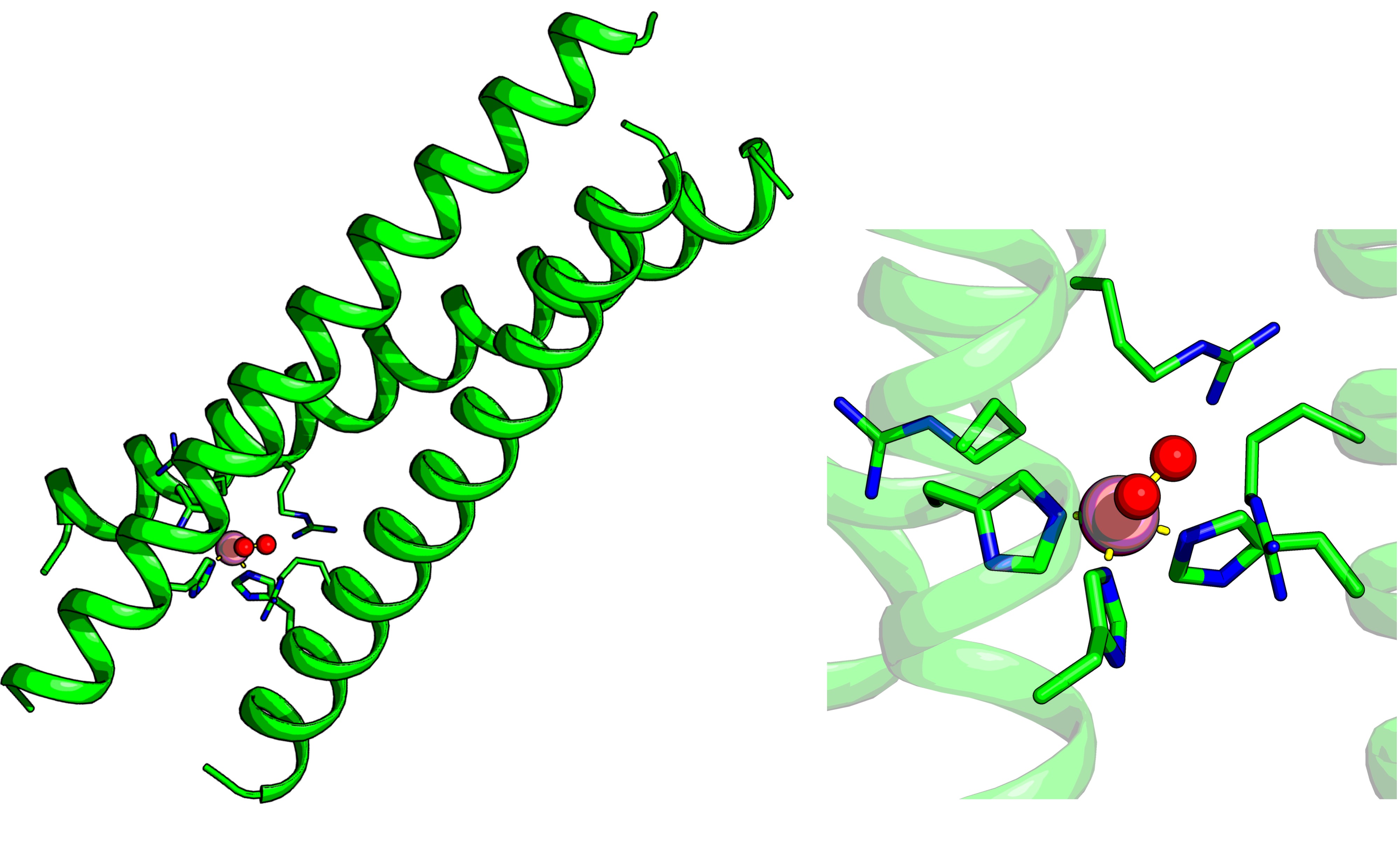
Figure 1. De novo designed three-stranded coiled coil model and close-up view of the cobalt-binding site.
- (1) Van Stappen, C.; Deng, Y.; Liu, Y.; Heidari, H.; Wang, J.-X.; Zhou, Y.; Ledray, A. P.; Lu, Y. Designing Artificial Metalloenzymes by Tuning of the Environment beyond the Primary Coordination Sphere. Chem. Rev. 2022, 122, 11974–12045.
- (2) La Gatta, S.; Pecoraro, V. L. Recent Advances in de Novo Designed Metallopeptides as Tailored Enzyme Mimics. Curr. Opin. Chem. Biol. 2025, 86, 102586.
- (3) Lancaster, K. M. Biological Outer-Sphere Coordination. In Molecular Electronic Structures of Transition Metal Complexes I; Mingos, D. M. P.; Day, P.; Dahl, J. P.; Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2012, 19–153.