Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Investigation of the DSF/Cu reaction using LC HDMS. (#518)
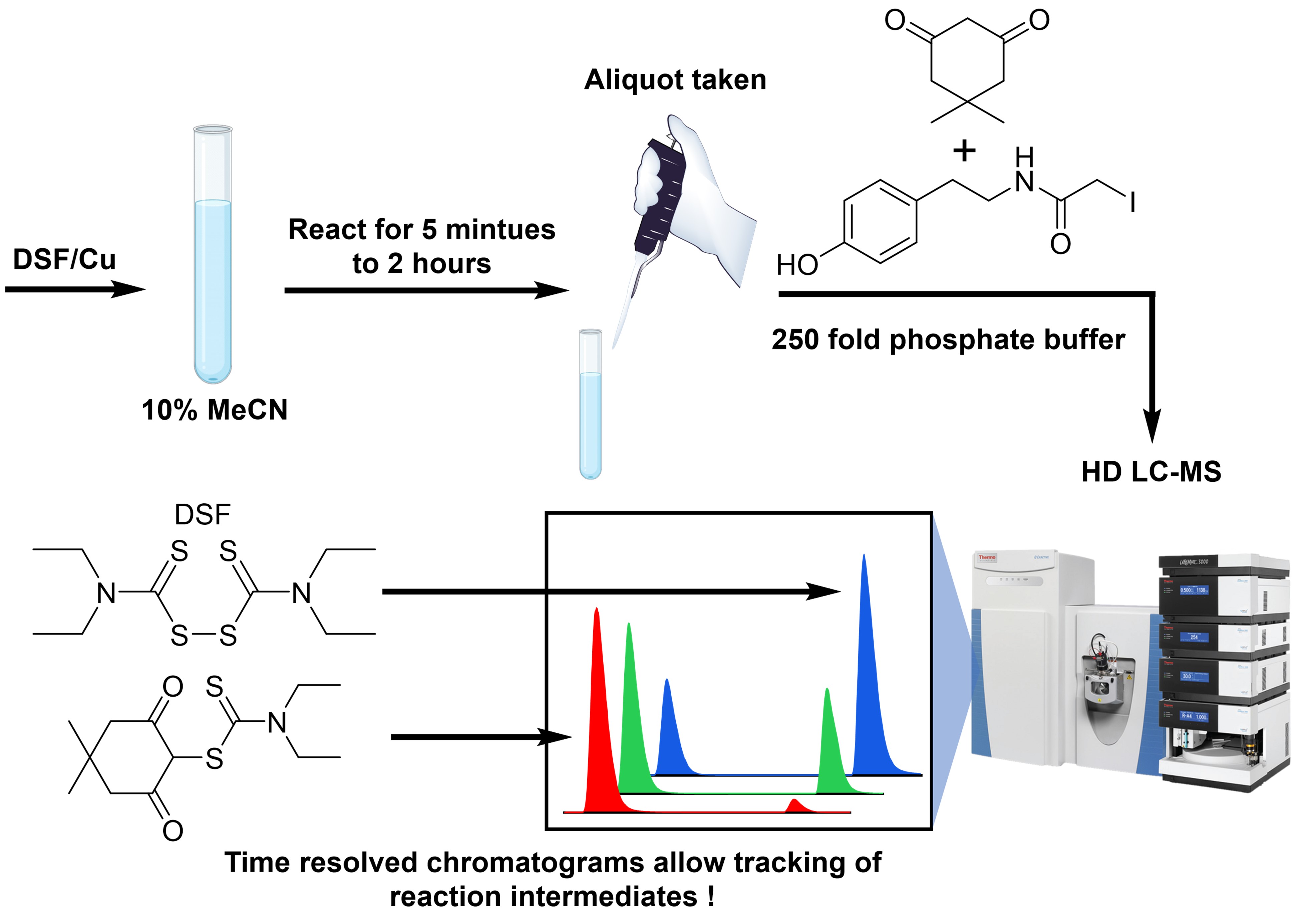
Cuprotosis is a unique mode of cell death being explored in many new therapeutic anticancer studies.[i] A widely used approach is the combination of Cu(II) salts with diethyldithiocarbamate disulfide, disulfiram (DSF), an alcohol-sensitizing drug Antabuse, which generates the toxic Cu(II)bis(diethyldithiocarbamate) complex, Cu(deDTC)2 in a complex redox reaction.[ii]. We have investigated the mechanism of the reaction by monitoring DSF/Cu(II) mixtures at different time points and conditions, using high-definition liquid chromatography-mass spectroscopy (HD LCMS) to determine intermediate products. Sulfur intermediates are further characterized by the addition of adduct-forming "traps" used to characterize thiol and sulfenic acid species generated throughout the reaction.[iii] Time-resolved data reveal the dominant DSF byproducts are formed by sulfur extrusion, oxidation, and insertion reactions leading to several reaction pathways for Cu(deDTC)2 formation. We intend this work to help inform new strategies for medicinal applications of cuprotosis.
[i] Chen, L.; Junxia, M.; Wang, F. "Copper homeostasis and cuproptosis in health and disease." Signal Transduction and Targeted Therapy 2022, 7, 378.
[ii] Cen, D.; Brayton, D.; Shahandeh, B.; Meyskens, F.L.; Farmer, P.J. "Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells." Journal of Medicinal Chemistry 2004, 47, 6914-6920.
[iii] Scrivner, O.; Kumar, M.; Sorokolet, K.; Wong, A.; Kebaara, B.; Farmer, P.J. "Characterization of endogenous and extruded H2S and small oxoacids of sulfur (SOS) in cell cultures." ACS Chemical Biology 2021, 16, 1413-1424.