Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Design, synthesis and biological evaluation of chemo-radiotheranostic chimera molecules fusing prostate targeting 99mTc complexes with potential antimitotic drugs (#511)
Radiotheranostic compounds are at the forefront of new advances in nuclear medicine for both diagnosis and treatment of cancers.[1] Whilst molecularly targeted radiotracer therapy shows great promise as an alternative treatment strategy, off-target dosimetry concerns mean that classic chemotherapy remains the primary standard of care.[2] Here, we aimed to combine the high sensitivity of gamma ray imaging with the controlled delivery of potent cytotoxic drug molecules by combining 99mTc complexes with both prostate specific lysine-urea-glutamate ligands and the antimitotic agent (R)-ispinesib to create a first-in-class chemo-radiotheranostic chimera.
The chimera molecule was designed and synthesized to incorporate a prostate specific membrane antigen (PSMA) targeting group, the anti-Eg5 drug (R)-ispinesib, attached via a cathepsin cleavable and self-immolative spacer (PABC-Cit-Val) and a norbornene as a ‘click’ handle to couple it to a TACN-99mTcO3+-core.[3, 4] Radiolabelling via ‘click’ reaction was conducted in water (80°C, pH 7-8). Cellular binding assays measured the IC50 using PC3-PIP(PSMA+) cells. Radiotracer pharmacokinetics and tumour targeting was evaluated in mice bearing subcutaneous PC3-PIP(+) xenograft using non-invasive planar γ-ray scintigraphy imaging, ex vivo biodistribution analysis and metabolite profiling with data compared against control compounds.
The bifunctional TACN-TcO-PSMA molecule (without (R)-ispinesib) was produced with an overall radiochemical yield of >30% (over two steps) and a radiochemical purity >99%. Cellular binding demonstrated specific localization to PC3-PIP cells. Imaging and biodistribution studies showed high tumor uptake (19.73±4.22 %ID/g after 4 hours) which was reduced by >80% in blocking control groups (3.16±0.62 %ID/g). Additionally, the TACN-TcO-PSMA-Ispinseib chimera molecule was successfully synthesized and fully characterized. Additional radiolabelling and biological tests are underway.
The TcO3-based labelling strategy is an ideal match labelling complex bioactive drug molecules and the resulting 99mTc-PSMA radiotracers were stable and showed high tumour-specificity both in vitro and in vivo. Combining 99mTc-click chemistry with cytotoxic drugs provides a new entry point toward chimeric chemo-radiotheranostic agents.
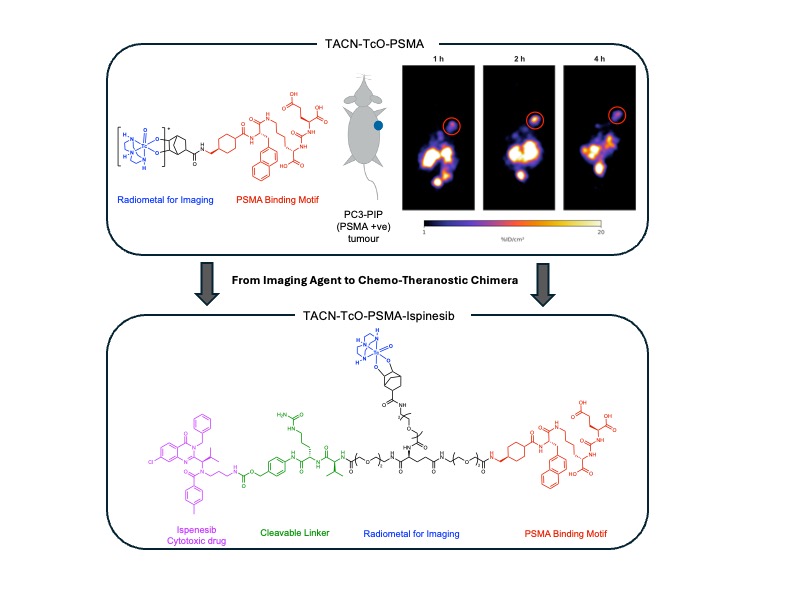
Fig. 1: Top: Structure of the bifunctional TACN-TcO-PSMA radiotracer with planar γ-ray scintigraphy images of tumour bearing mice. Bottom: Structure of the Chemo-Theranostic Chimera TACN-TcO-PSMA-Ispinesib.
- Bodei, L., Herrmann, K., Schöder, H. et al., Nat Rev Clin Oncol, 2022, 19, 534–550.
- Chigoho, D. M., Bridoux, J. & Hernot, S. Current Opinion in Chemical Biology, 2021, 63, 219–228.
- Dubowchik, G. M. et al. Bioconjugate Chem., 2002, 13, 855–869.
- Braband, H., Tooyama, Y., Fox, T. & Alberto, R. Chem. Eur. J, 2009, 15, 633–638.