Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
From stable to labile: Fine-tuning the off-target uptake of radiotracers (#510)
One of the major challenges of molecularly targeted radiotherapy is to reduce off-target uptake of the radioconjugates in the healthy organs. We modified our radiotracer designs to incorporate enzymatically cleavable linkers that help eliminate the radioactivity from background tissues via controlled tissue-specific metabolism. Proof-of-concept models were tested with the monoclonal antibody onartuzumab and a lys-urea-glutamate radiotracer targeting the prostate-specific membrane antigen (PSMA) to address dose limiting kidney uptake.[1,2]
68Ga and 177Lu radiotracers bound to the PSMA-targeting unit or to onartuzumab were designed to incorporate the MWK tripeptide, a known substrate for neprilysin expressed in the kidney. Stability studies were tested in vitro using homogenised porcine renal cortex. Radiotracer studies in mice bearing either PC-3-PIP (for PSMA studies) or MKN-45 xenografts (expressing c-MET for onartuzumab experiments) were performed using a combination of non-invasive positron emission tomography, gamma-ray scintigraphy, ex vivo biodistribution analysis and HPLC analysis of biological fluids.
Both the 68Ga-DOTA-MWK-PSMA and 177Lu-DOTAGA-MWK-onartuzumab were prepared with high radiochemical yields, radiochemical purity, and molar activities. In vitro assays indicated high stability of the conjugates in the absence of enzymes or tissue lysates. Imaging and biodistribution experiments with 177Lu-DOTAGA-MWK-onartuzumab showed high and specific tumour uptake (41.9±7.5%ID/g at 72h, figure 1) but also showed high accumulation in the kidney. While the radioconjugate underwent rapid decomposition in tissue lysate in vitro, no renal excretion was observed in vivo. For 68Ga-DOTA-MWK-PSMA high and specific tumour uptake was observed (12.1±3.5%ID/g at 4h) with PET image contrast improving out to 4 hours. HPLC analysis of urine samples revealed one new metabolite peak which was confirmed by independent synthesis to be the MWK cleavable byproduct.
Whilst the MWK peptide sequence proved effective at modulating the pharmacokinetic profile of a 68Ga-PSMA-targeted small-molecule, it failed to enhance kidney clearance using the 177Lu-antibody model. Future studies will explore alternative cleavable-linker/enzyme combinations.
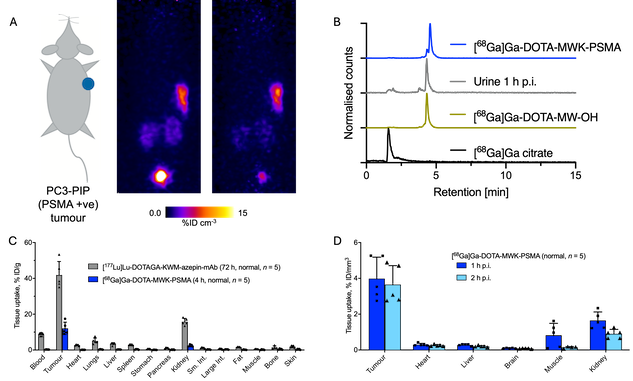
Figure 1. A: PET of a tumour-bearing mouse at 1h and 2h p.i. B: HPLC analysis of the tracer, urine metabolites, suspected metabolite, and 68Ga. C: Ex vivo biodistribution of 177Lu-DOTAGA-MWK-onartuzumab (72h p.i.) and 68Ga-DOTA-MWK-PSMA (4h p.i.). D: In vivo distribution of 68Ga-DOTA-MWK-PSMA at 1h and 2h p.i., from PET images.
- Schäfer, H.; Mayr, S.; Büttner-Herold, M.; Knorr, K.; Steinhelfer, L.; Böger, C. A.; Gschwend, J. E.; Heemann, U.; Eiber, M.; Schmaderer, C.; Tauber, R. Eur. Urol., 2023, 83 (5), 385–390.
- Klingler, S.; Fay, R.; Holland J. P. J. Nucl. Med., 2020, 61 (7), 1072-1078.