Invited Talk 21st International Conference on Biological Inorganic Chemistry 2025
Effect of Structural Distortion on the Oxidation Reactivity of a Di(μ-oxido)dinickel(III) Complex (#42)
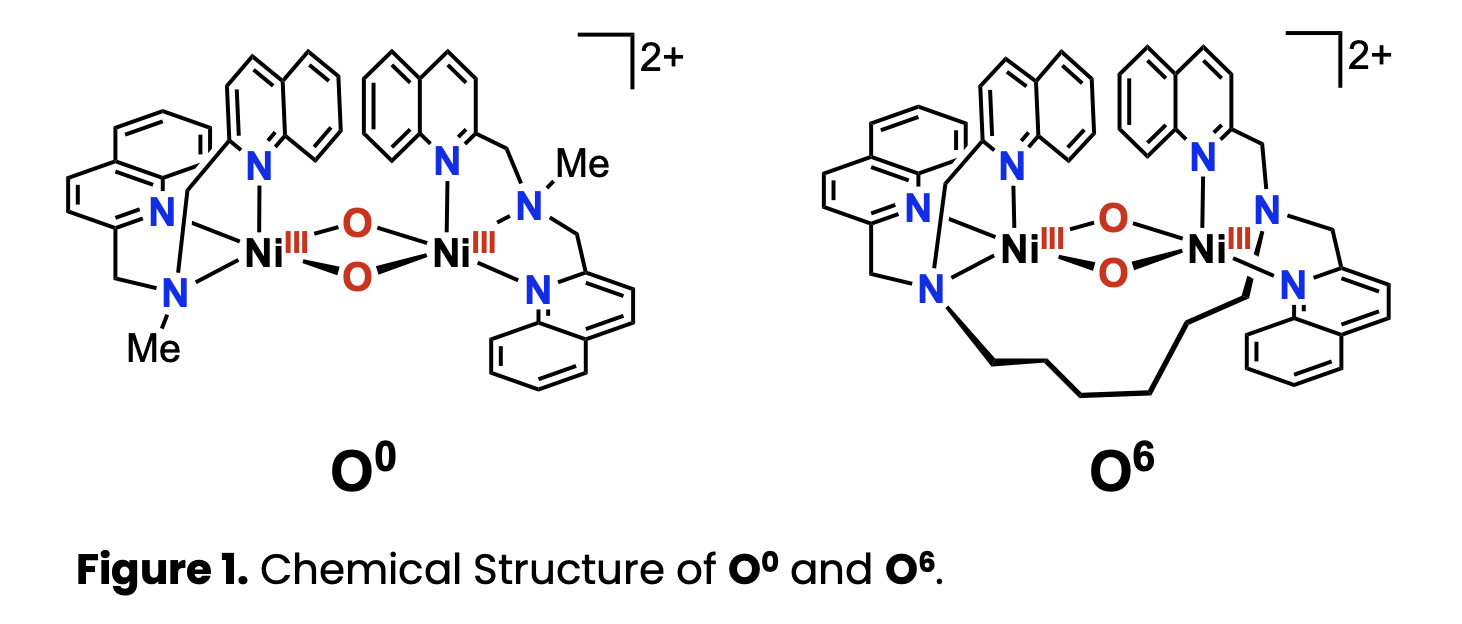
In metalloenzymes, protein framework with rigidity often distorts coordination bonds between the metal center and amino acid residues from their most stable geometries. In highly active oxygenase enzymes, such structural distortion is believed to play a critical role not only in appropriately modulating the chemical potentials of both the resting state and the reactive intermediate, but also in minimizing the structural rearrangement required during the transition between these states. Such purposefully distorted structures are referred to as the entatic state. We have investigated functional models of oxygenase enzymes with dinuclear metal centers, such as soluble methane monooxygenase (sMMO) and tyrosinase. In this context, we reported the isolation and characterization of a di(μ-oxido)dinickel(III) complex (O0) bearing quinolylamine ligands, along with its reactivity (Figure 1).[1] The present study aims to artificially induce an entatic state in dinuclear metal complexes.
Hexadentate ligands (L6), in which bisquinolylmethylamine ligand L0 was tethered by hexylene linker, was synthesized. Di(µ-oxido)dinickel(II) complexes (OH6) were then prepared. The formation of O6 was observed upon addition of an excess amount of H₂O₂ at –80 °C (Figure 1). Under these conditions, the OH0 complex, which lacks a bridging moiety, did not produce O0.
Subsequently, reactivity of these complexes was kinetically investigated using 9,10-dihydroanthracene (DHA) as a substrate, and second-order reaction rate constants (k, M–1 s–1) at –60 °C were determined. A comparison of k for O6 [1.2 × 10–1 M⁻¹ s⁻¹] with that for O0 [8.8 × 10⁻³ M⁻¹s⁻¹].
The introduction of the linker accelerated both the formation of the reactive species and its reaction with the substrate.
- [1] Morimoto, Y. et al. Angew. Chem., Int. Ed. 2018, 57, 7640