Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Targeting Peptide Stability: GLP-1 analogues, metal-peptide complexes and enzymatic stability. (#508)
Glucagon-like peptide-1 (GLP-1) is an endogenous incretin peptide hormone that plays a key role in regulating blood glucose levels. Secreted in response to food intake, it stimulates insulin secretion in a glucose-dependent manner, inhibits glucagon release, and slows gastric emptying1,2,3. In patients with type 2 diabetes, the incretin effect—primarily mediated by GLP-1—is significantly impaired4. Despite its beneficial biological properties, the therapeutic application of native GLP-1 is limited due to its rapid enzymatic degradation by dipeptidyl peptidase IV (DPP-4) and neprilysin (NEP)5. To overcome these limitations, GLP-1 analogues such as liraglutide have been developed, exhibiting increased metabolic stability.
To investigate the coordination behavior of Cu(II) and Zn(II) ions with selected peptides—where Zn(II) is of particular importance in the context of type 2 diabetes due to its role in the biochemistry of insulin and glucagon6 — native GLP-1 (HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG), liraglutide (HAEGTFTSDVSSYLEGQAAK(Pal-γE)EFIAWLVRGRG), and a modified version of liraglutide (HaEGTFTSdVSsyLEGQAAK(Pal-γE)efIAwLVRGRG) in which L-amino acids at enzymatically labile sites were substituted with their D-enantiomers to enhance resistance to DPP-4 and NEP-mediated cleavage (Figure 1.).
Potentiometric titrations, UV-Vis absorption spectroscopy, and circular dichroism (CD) spectroscopy were employed to characterize metal–peptide interactions. Additionally, high-performance liquid chromatography (HPLC) was used to assess the enzymatic stability of GLP-1 and its analogues.
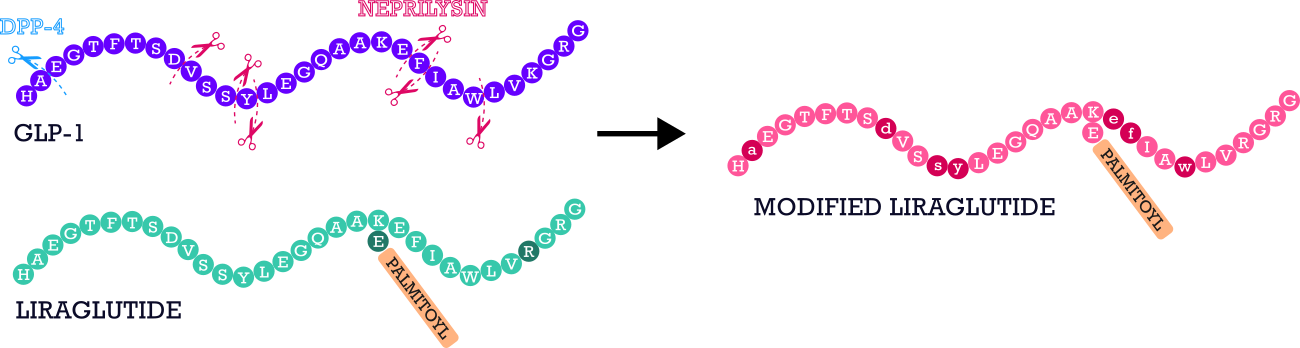
Fig. 1 Sequences of the studied peptides: GLP-1 with marked cleavage sites by DPP-4 and neprilysin, liraglutide, and modified liraglutide.
- J. Holst, J. Gromada, American Journal of Physiology-Endocrinology and Metabolism, 2004, 287, E199–E206
- C. Orskov, J. J. Holst and O. V. Nielsen, Endocrinology, 1988, 123, 2009–2013
- A. Wettergren, B. Schjoldager, P. E. Mortensen, J. Myhre, J. Christiansen,J. J. Holst, Digestive Diseases and Sciences, 1993, 38, 665–673
- A. Nauck, J. J. Meier, Diabetes, Obesity and Metabolism, 2018, 20, 5-21
- F. Deacon, Hormone and Metabolic Research, 2004, 36, 761–765.
- W. Maret, Preventive Nutrition and Food Science., 2017, 22(1), 1-8.