Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
A μ-1,1-Nitroso Dicopper complex with unusual electronic structure and its one electron reduction triggering NO release (#598)
A μ-1,1-Nitroso Dicopper complex with unusual electronic structure and its one electron reduction triggering NO release
Metal-mediated activation of nitric oxide leads to reactive intermediates that play crucial roles in physiological functions, ranging from neurotransmission to immune response.1,2 Synthetic model complexes provide valuable insights into the intricate electronic structures of metal nitrosyls, which are essential for understanding their distinct properties.3 However, the number of structurally characterized Cu/NO complexes is still very limited.
In this study we introduce a novel {Cu2(NO)}20 type complex [LCu2(NO)]2+ (12+; Scheme 1), synthesized by reacting a dicopper(I) complex (2⁺) supported by a dinucleating pyrazolate/tacn ligand L− (tacn = 1,4,7-triazacyclononane) with NO⁺. The complex exhibits an unusual electronic structure and reactivity, shedding light on biorelevant Cu/NO interactions.4 One-electron reduction of 12+ yields a transient {Cu₂(NO)}21 intermediate (1+), which is unstable even at low temperatures. 1+ can also be formed through an alternative pathway from 2⁺ and NO gas. This process first generates the intermediate {Cu₂(NO)}21 (1+), which then converts to 12+. Spectroscopic and computational studies reveal an intriguing electronic structure of 1⁺, reflected in a distinct absorption feature in the visible region. This intermediate 1+ readily releases NO, regenerating the dicopper(I) complex (2⁺), which under aerobic conditions reacts with O₂ to form the known peroxo-bridged species [LCuII2(μ1,2-O2)]+;5 the same final product is formed upon reaction of 12+ with superoxide. This work provides key insight in the electronic structure of the {Cu2(μ1,1-NO)} core in different oxidation states, as a basis for understanding biologically relevant Cu/NO interactions and reactivities.
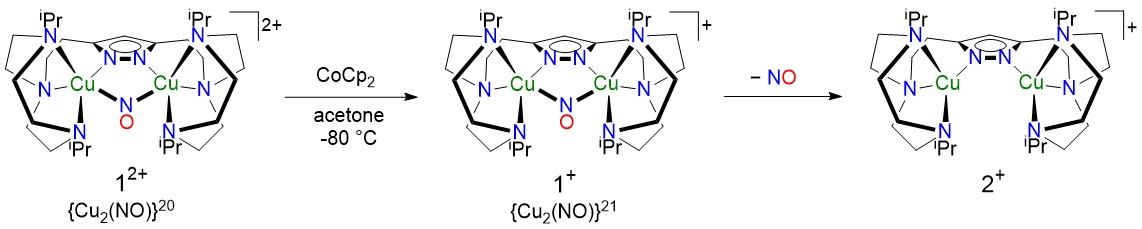
Scheme 1 Cu2(NO)}20 complex 12+ and its reduction followed by NO release
[1] Butler A. R.; Williams D. L. H. Chem. Soc. Rev., 1993, 22, 233.
[2] Bogdan, C. Nat. Immunol., 2001, 2, 907.
[3] Hayton, T. W.; Legzdins, P.; Sharp, W. B. Chem. Rev., 2002, 102, 935.
[4] Spyra, C. J.; Chen, H.; Bhattacharya, D.; Wojcik, L.; Dechert, S.; Poul, N. Le.; Ye, S.; Meyer, F. Inorg. Chem., in revision.
[5] Kindermann, N.; Bill, E.; Dechert, S.; Demeshko, S.; Reijerse, E. J.; Meyer, F. Angew. Chem. Int. Ed., 2015, 54, 1738.
- [1] Butler A. R.; Williams D. L. H. Chem. Soc. Rev., 1993, 22, 233.
- [2] Bogdan, C. Nat. Immunol., 2001, 2, 907.
- [3] Hayton, T. W.; Legzdins, P.; Sharp, W. B. Chem. Rev., 2002, 102, 935.
- [4] Spyra, C. J.; Chen, H.; Bhattacharya, D.; Wojcik, L.; Dechert, S.; Poul, N. Le.; Ye, S.; Meyer, F. Inorg. Chem., in revision.
- [5] Kindermann, N.; Bill, E.; Dechert, S.; Demeshko, S.; Reijerse, E. J.; Meyer, F. Angew. Chem. Int. Ed., 2015, 54, 1738.