Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Synthesis of a mimetic of the lytic polysaccharide monooxygenase (LPMO) enzyme for catalyzing the oxidative hydrolysis reaction of cellulose (#462)
A mimetic of the LPMO enzyme, a mononuclear copper enzyme coordinated to two histidine residues, was synthesized to study its catalytic activity in polysaccharide segregation for non-polluting energy production from biomass via oxidative hydrolysis reactions1.
The project involved four organic synthesis steps to obtain the ligand, followed by copper coordination. The ligand was synthesized from L-proline esterification, generating product (1). Alkylation of (1) with bromomethyl pyridine yielded compound (2), which was hydrolyzed under alkaline conditions to obtain (3). Coupling (3) with an anthranilic ester produced ligand (LH), coordinated with Cu²⁺ to form [CuL].
Complex [CuL] was characterized by UV-vis and FTIR spectrophotometry, microanalysis, cyclic voltammetry, High-Resolution Mass Spectroscopy and EPR spectroscopy. The UV-vis spectrum revealed non-linearity of the molar absorptivity upon increase in concentration, evidencing a dynamic equilibrium between species in solution. EPR spectroscopy revealed two coexisting complexes in equimolar concentrations. The increase in the solution pH favors one of them, indicating that its formation occurs via deprotonation/protonation step. Catalytic tests were carried out using colorimetric methods with p-nitrophenyl-β-D-glucopyranoside as a cellulose mimetic2. The catalytic efficiency increased due to the higher concentration of oxidizing agent, H₂O₂, in the reaction. The kinetic constant at 1.6 mM H₂O₂ was 1.96 x 10-³ s-¹, while at 10 mM H₂O₂ it was 2.4 x 10-³ s-¹ (Figure 1).
The [CuL] complex exhibited promising catalytic activity in oxidative polysaccharide hydrolysis. The presence of two species suggests a pH-dependent equilibrium affecting its catalytic potential. To improve system stability, new syntheses with modified ligands were initiated to assess the influence of the carboxyl group and steric hindrance on coordination and reactivity. Future studies will explore catalytic tests with CO₂ as a co-substrate to further evaluate [CuL]’s role in polysaccharide degradation.
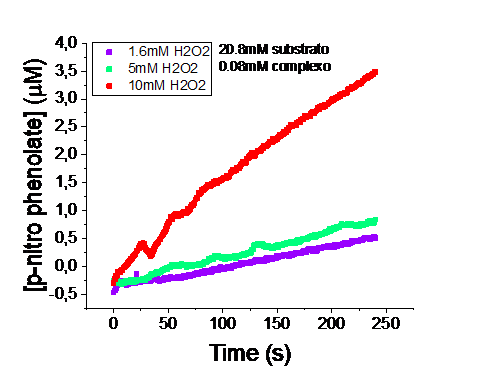
Figure 1. Comparison of the catalytic activity kinetics of [CuL] using colorimetric methods with p-nitrophenyl-β-D-glucopyranoside.
- (1) Bell, E. L.; Finnigan, W.; France, S. P.; Green, A. P.; Hayes, M. A.; Hepworth, L. J.; Lovelock, S. L.; Niikura, H.; Osuna, S.; Romero, E.; Ryan, K. S.; Turner, N. J.; Flitsch, S. L. Biocatalysis, Nature Reviews Methods Primers, 2021, 1, 46.
- (2) Concia, A. L.; Beccia, M. R.; Orio, M.; Ferre, F. T.; Scarpellini, M.; Biaso, F.; Guigliarelli, B.; Réglier, M.; Simaan, A. J. Copper complexes as bioinspired models for lytic polysaccharide monooxygenases, Inorganic Chemistry, 2017, 56 (3), 1023-1026.