Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Computational Study on Active Site Residues Impact in Au(I) Complex Reactivity with Trypanothione Reductase (#458)
Gold complexes have been studied as drugs since Robert Koch and Jacques Forrestier’s discoveries1. Beyond arthritis treatment, they show potential as antiparasitic agents by inhibiting key enzymes via thiol and selenol residues, due to their thiophilic nature1. Our group investigates Au(I) complexes as potential leishmanicidal agents, targeting trypanothione reductase (TR), a redox enzyme reliant on active-site cysteines. Most DFT studies on this topic use single-molecule models, including our previous work and Tolbatov’s2-4. While our calculations of cysteine exchange with Au(I)-NHC complexes align with experiments, docking and molecular dynamics lack the quantum mechanical accuracy needed for metal-enzyme reactions. Here, we refine our approach by investigating Au(I)-TR interactions using a detailed model incorporating key active site residues.
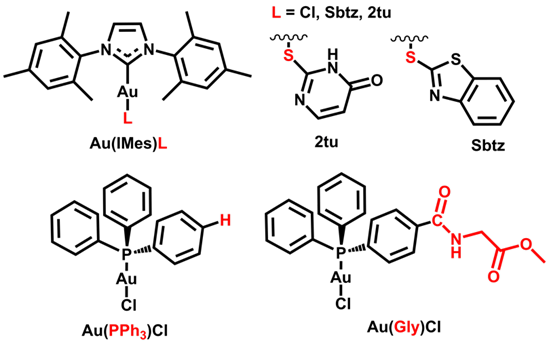
Figure 1. Structures of studied complexes
The studied complexes include Au(NHC) with Cl⁻ and thiolates (Au(IMes)L, L = Cl, benzothaizolidine, 2-thiouracyl) and Au-phosphine, one with triphenylphosphine and another with a phosphine containing a glycine fragment (Figure 1). Structures were optimized using DFT (PBE0, ZORA-def2-TZVP, CPCM/water), and molecular docking (GOLD) was performed for Au(I) complexes with TR (PDB: 1BZL) to obtain initial geometries. The system was then truncated to a dipeptide model (Cys53–Cys58, His461–Glu466) (Figure 2). The Gibbs free energy variation for ligand exchange at Cys58 was calculated using xTB for each complex (Table 1).
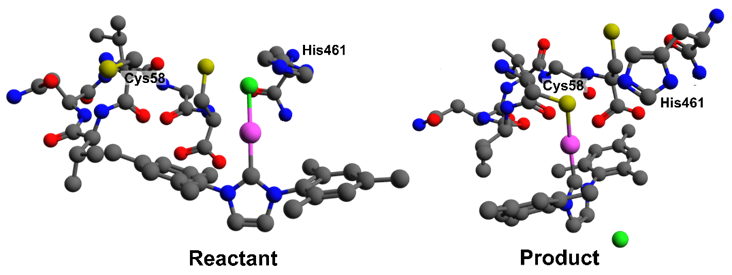
Figure 2. Dipeptide Model Reactant and Product structures for Au(IMes)Cl. Pro462-Glu466 and hydrogens omitted for clarity.
Table 1. ΔG for the reactions, calculated based on each model.
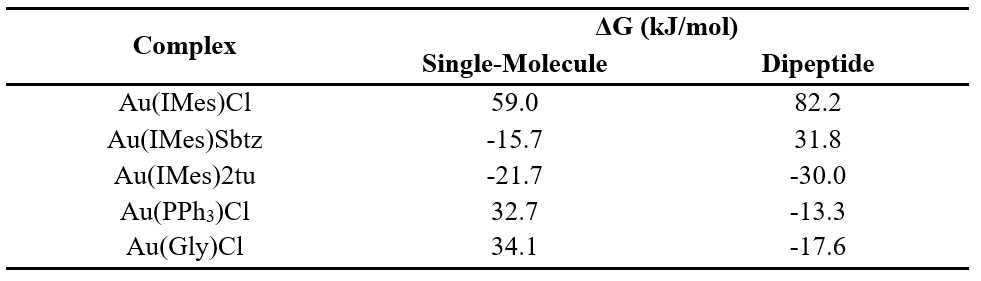
The proposed model structures revealed new intermolecular interactions that affect the complexes’ reactivity. In the product structures, the free 2tu ligand forms hydrogen bonds with Glu466, stabilizing the product, Sbtz, due to steric effects, become endergonic. Au(PPh3)Cl and Au(Gly)Cl both present similar reactivity in the single-molecule model, but AuGly is more reactive in the dipeptide model due to stabilizing interactions present in AuGly product (Ser464).
We can conclude that enzyme active-site residues affect reactivity. Further, other phosphine complexes are being studied, and more robust DFT calculations are underway to enhance accuracy.
- Beners-Price, S. J. et al. Metallomics, 2011, 3, 863-873.
- Rodrigues, G. C et al. New Journal of Chemistry, 2024, 48, 2040-2047.
- Oliveira, S. I. et al. Dalton Transactions, 2024, 53, 18963-18973.
- Tolbatov, I. et al. Inorganic Chemistry, 2020, 59, 3312-3320.