Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Construction of myoglobin dimers by designed 3D domain swapping (#449)
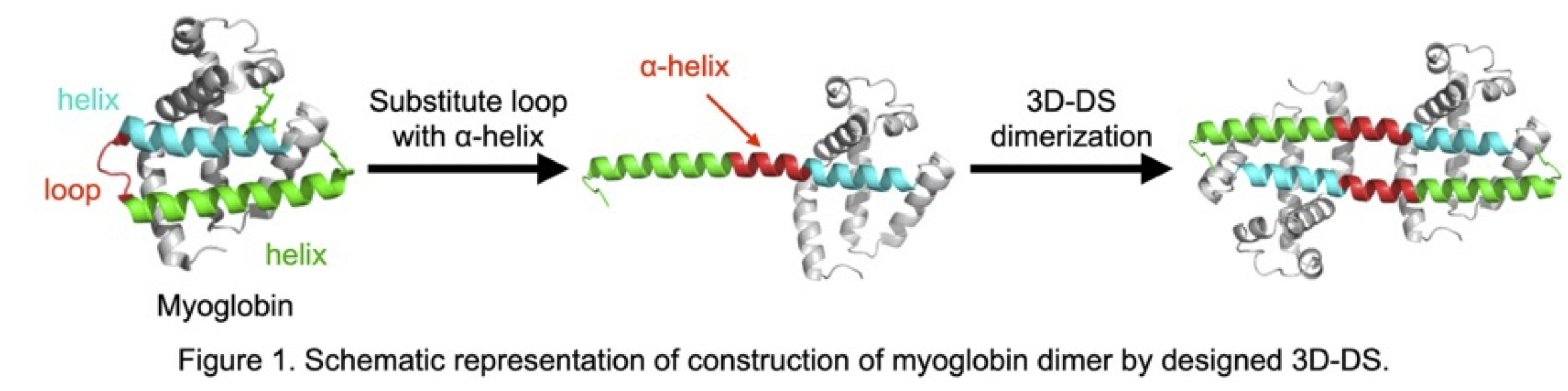
In nature, protein assemblies play important roles in biomolecule production, storage, transfer, etc. Recently, protein assemblies have been constructed as new functional biomaterials, increasing the importance of oligomerization methods that can stably form desired protein oligomeric structures. We previously showed that myoglobin (Mb), which consists of eight α-helices (A–H), dimerizes via 3D domain swapping (3D-DS) wherein the same structural region is exchanged three-dimensionally between molecules of the same protein. The hinge loop between helices E and F converted to a helical structure and merged with helices E and F to form one extended α-helix.1 In this study, we constructed stable Mb dimers with 3D-DS. We aimed to induce dimerization through 3D-DS by modifying a helix-loop-helix motif into a single continuous helix at a target site. Specifically, mutations were introduced into the GH loop region of Mb (Figure 1). 21 variants of Mb (Mb1–Mb21), in which the GH loop region was replaced with reported α-helical linkers, were designed and two dimeric variants, Mb3 and Mb8 were successfully obtained. X-ray crystallographic analysis revealed that the GH loop region in both variants form an α-helix, leading to dimerization via 3D-DS. However, both variants showed reduced thermal stability compared to that of wild-type Mb and existed in equilibrium between monomeric and dimeric forms. To stabilize the dimeric structure, the surface and the inserted helical linker segments of Mb3 were redesigned with ProteinMPNN based on the crystal structure of the Mb3 dimer. Six redesigned Mb variants (reMb3A–F) were expressed in E. coli, and five of them were obtained exclusively as dimers as soluble components, with higher yields than wild-type Mb. reMb3B, which showed the highest expression level, was analyzed by X-ray crystallography, revealing a dimeric structure similar to that of Mb3. In addition, the melting temperature of reMb3B was higher than that of Mb3 for more than 20 °C, indicating a significant improvement in thermal stability. In this presentation, we will also discuss the formation of higher-order Mb oligomers via continuous 3D-DS, constructed by combining designed 3D-DS at the EF-loop region.
- 1. S. Hirota et al., Dalton Trans., 2012, 41, 11378–11385.