Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Tuning of Redox Potential for the Electrochemical reduction of Nitrite to Nitric Oxide by tripodal amine based copper complexes for Biomedical applications (#426)
Nitric Oxide (NO) is an essential endogenous molecule involved in numerous physiological processes, including vasodilation, immune response, and inhibition of platelet aggregation. The incorporation of NO-releasing capabilities into intravenous (IV) catheters presents a promising therapeutic strategy to mitigate the risks of thrombosis and bacterial infections associated with vascular access. Our collaborative research with Prof. Meyerhoff has focused on the development of IV catheters capable of controlled NO release via the electrochemical reduction of nitrite, a cost-effective, stable, and non-toxic NO precursor, using novel Cu(II)-L catalyst mediators.
To optimize ligand structures and catalytic performance, a series of six Cu(II)-based catalysts were investigated, including Cu(II)-BMPA, Cu(II)-BEPA, and their carboxylate-appended variants (Et, Pr, and Bu). Despite advancements in catalyst longevity, NO flux, and antimicrobial efficacy, limitations remained, particularly in oxygen sensitivity due to the catalysts' negative reduction potential. When transitioning from a 100% nitrogen environment to a gas mixture containing 10% oxygen, NO flux exhibited a substantial decline. To address this, we explored ligand modifications by replacing pyridine with quinoline, inspired by Prof. Karlin’s extensive studies on Cu-oxygen chemistry. Additionally, a new set of ligands incorporating pyrazole and quinoline moieties was synthesized, forming Cu(II) complexes with various salts, including Cu(OAc)₂, Cu(ClO₄)₂, and CuSO₄. These complexes were characterized using multiple spectroscopic techniques, and their redox properties were evaluated via cyclic voltammetry. The efficiency of NO generation through the electrochemical reduction of nitrite was quantified using a NOA instrument, with promising preliminary results.
Further, alternative ligand designs based on TACN (1,4,7-triazacyclononane) were explored to enhance electrochemical nitrite reduction under physiological conditions. Two new Cu(II) catalysts were synthesized and tested, demonstrating significant potential for NO generation in IV catheters. These ongoing investigations aim to optimize catalytic performance, improve oxygen tolerance, and enhance the clinical applicability of NO-releasing IV catheters.
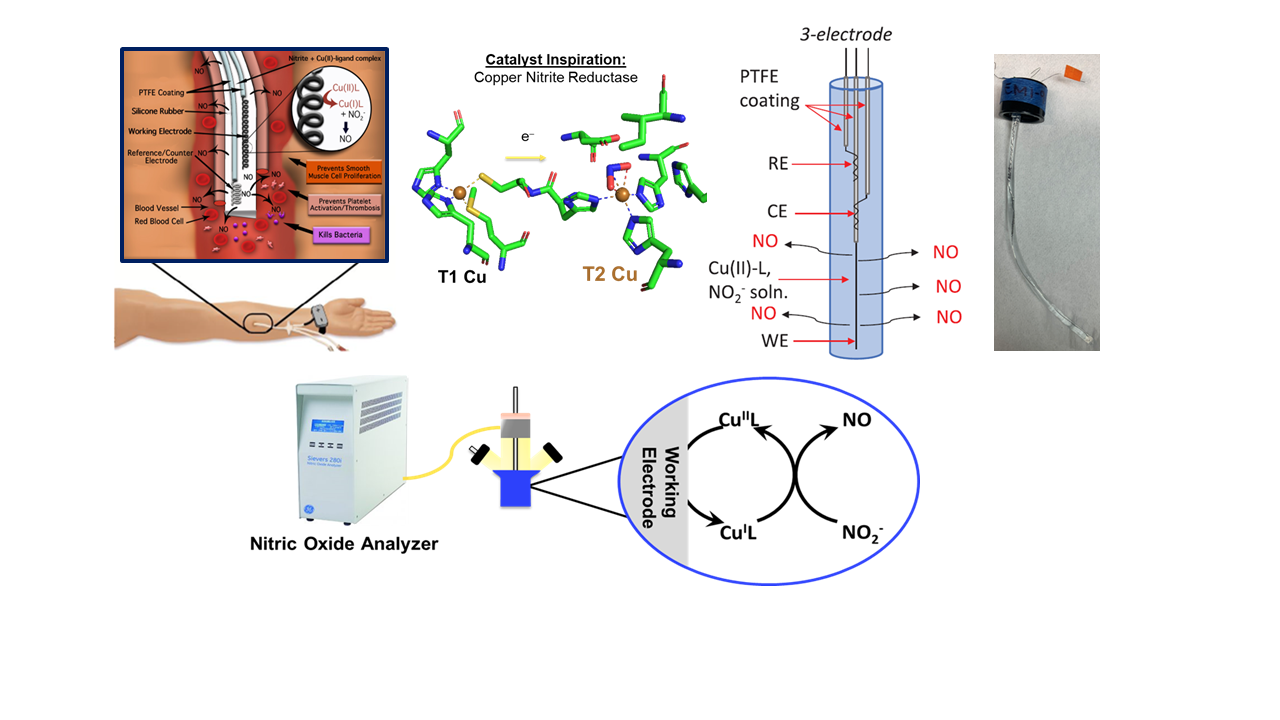
- CJ White, JM Schwartz, N Lehnert, ME Meyerhoff Bioelectrochemistry, 2023 152, 108448
- Andrew P Hunt, Allison E Batka, Marjan Hosseinzadeh, Jordan D Gregory, Halima K Haque, Hang Ren, Mark E Meyerhoff, Nicolai Lehnert ACS catalysis 2019 9 (9), 7746-7758