Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Nitric Oxide Binding to the Synthetic Heme Protein Model, "hemoCD", Composed of Iron Porphyrin Encapsulated in the Cyclodextrin Nanocavities in Water. (#425)
Nitric oxide (NO) has been recognized as a gas transmitter which has biological functions such as vasodilation in mammalian body. Since the discovery of the binding of NO to heme in soluble guanylate cyclase (sGC), the interaction between NO and heme (including synthetic heme models) has been studied extensively to understand their biological reactions.
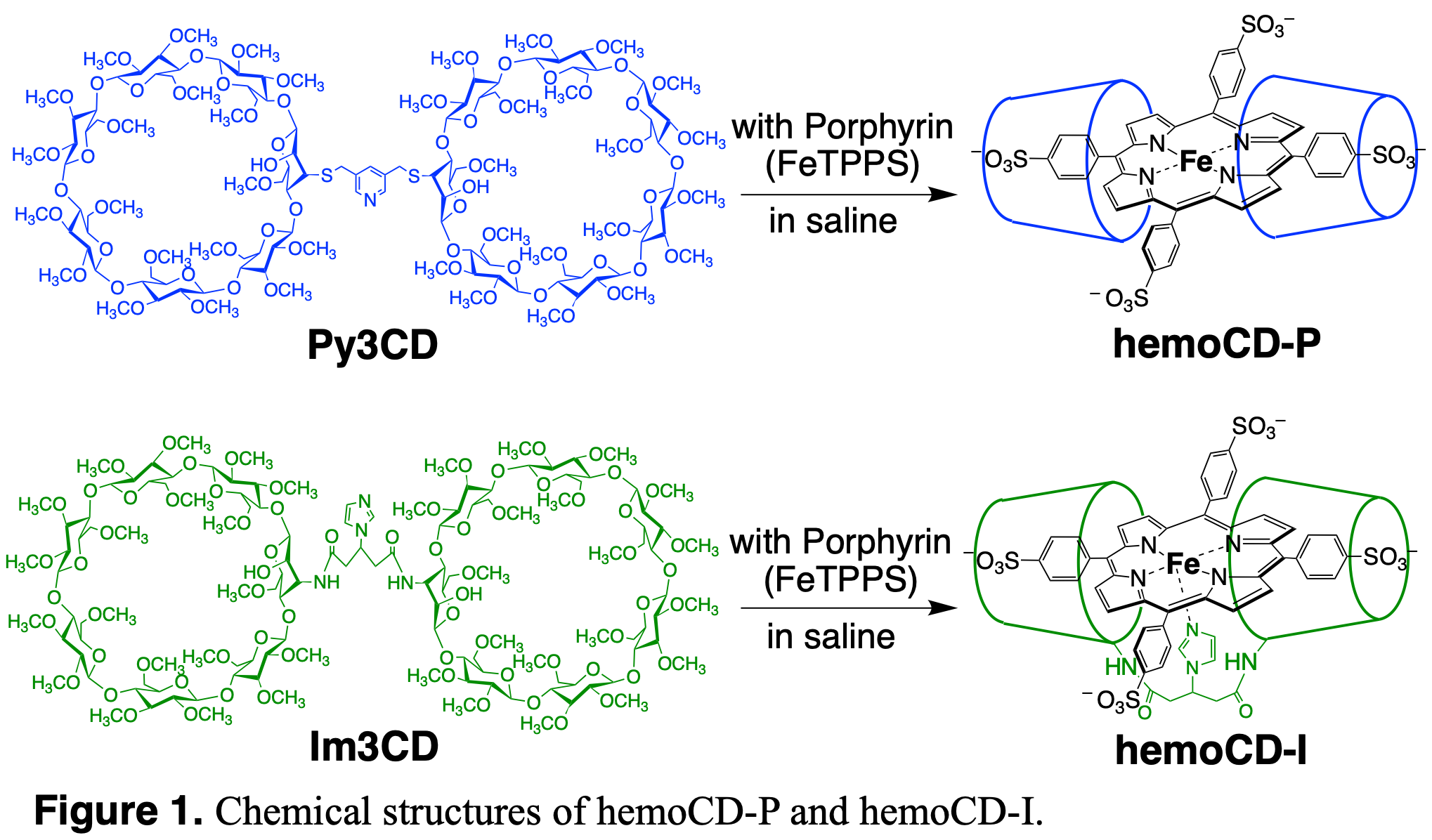
Our group constructed a synthetic heme protein model, “hemoCD”, which is composed of iron porphyrin (FeTPPS) and linkered O-methylated β-cyclodextrin (CD) dimer.1 Encapsulation by methylated cyclodextrins provide the microscopic hydrophobic environment for FeTPPS, isolating from aqueous phase. Since this effect is exactly same as what globin protein does in heme protein, our hemoCD successfully mimics heme proteins’ function in aqueous media also in vivo. We synthesized two types of hemoCD: hemoCD-P with a pyridine linker and hemoCD-I with an imidazole linker (Figure 1). Using hemoCDs, we have conducted systematic studies on the interaction with gaseous molecules such as carbon monoxide, hydrogen sulfide and hydrogen cyanide. Furthermore, we examined their interactions in living cells and in animal body, proving the effectiveness of hemoCDs as ready-to-use antidotes against those poisonous molecules in vivo.2,3 However, the study on NO has been remaining unrevealed due to its high reactivity and complexity.
In this study, we carried out the detailed study on the interaction of hemoCDs towards NO, including the characterization of their nitrosyl complexes, thermodynamic and kinetic properties and reductive nitrosylation in water. We showed the formation of both ferric and ferrous nitrosyl hemoCD complexes by UV-vis, resonance raman and EPR spectroscopies. The binding affinities between hemoCDs and NO were comparable to native heme proteins. Also, the structural difference between hemoCD-P and -I was clearly demonstrated through reductive nitrosylation. Finally, we summarize affinities of major gaseous molecules in hemoCD, which leads us to a conclusion that hemoCDs are sophisticated biomimetic heme protein models in water.
- (1) Kitagishi, H.; Kano, K. Chem. Commun., 2021, 57, 148-173.
- (2) Mao, Q.; Zhao, A.; Kiriyama, A.; Negi, S.; Fukuda, Y.; Yoshioka, H.; Kawaguchi, A. T.; Motterlini, R.; Foresti, R.; Kitagishi, H. Proc. Natl. Acad. Sci. USA., 2023, 120, e2209924120.
- (3) Nakagami, A.; Mao, Q.; Horitani, M.; Kodera, M.; Kitagishi, H. Sci. Rep., 2024, 14, 29371.