Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Eu(II)EGTA in Hydrogenase Kinetics: Improved Rates and New Insights into pH and Solution Potential dependencies (#422)
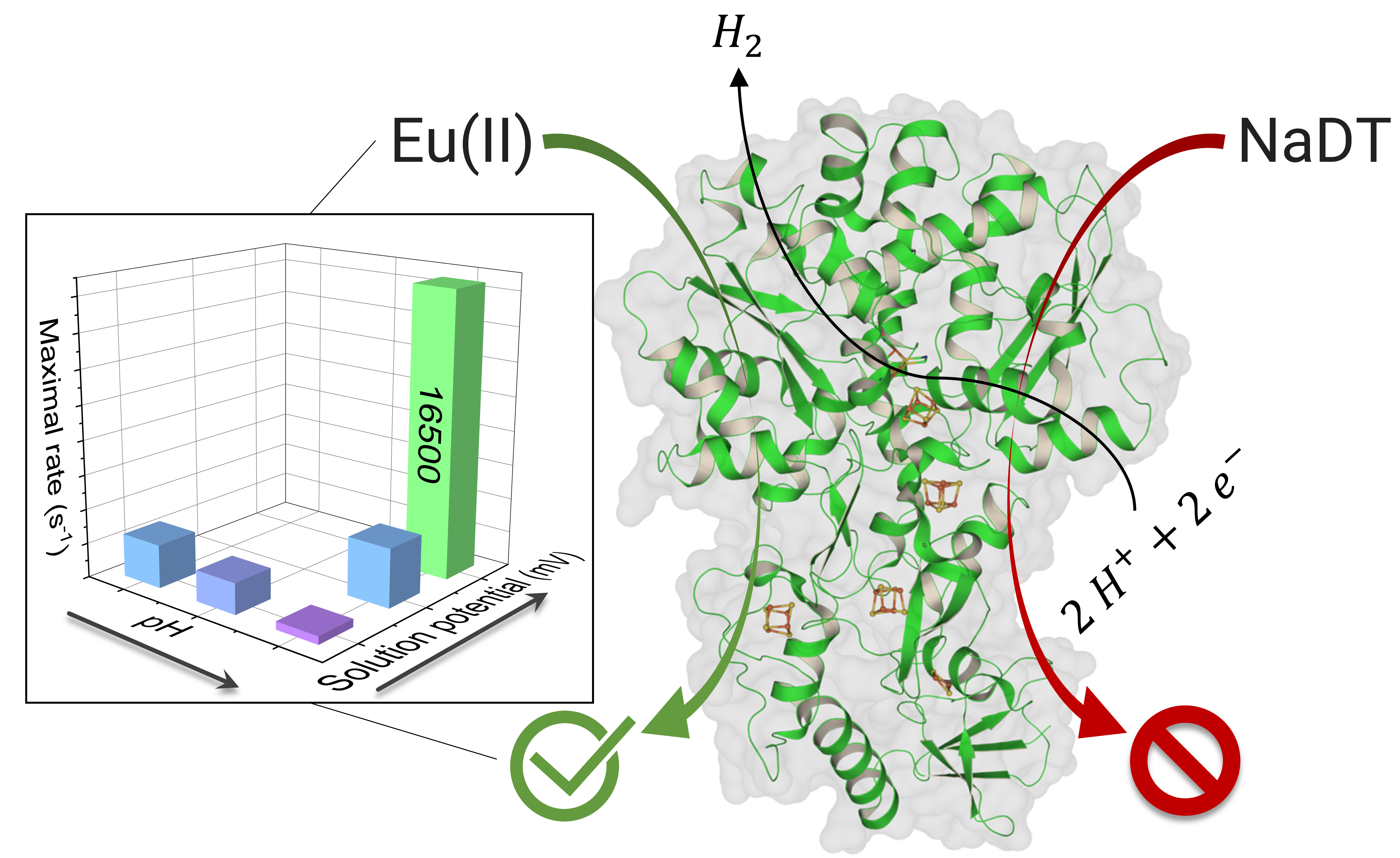
Metalloenzymes such as nitrogenase, carbon monoxide dehydrogenase, and hydrogenases are central to microbial processing of N₂, CO₂, and H₂. Despite their efficiencies and biotechnological relevance, the mechanistic details of these enzymes remain unclear. Here I will present our recent efforts aimed at identifying the causes of discrepancies in kinetic investigations of hydrogenases, and the development of solution assays employing Eu2+ as the terminal electron donor instead of the commonly used, but problematic, reductant dithionite (NaDT).
Kinetic studies were done using [FeFe] hydrogenase I from Clostridium pasteurianum (CpI) as a model system. Solution assays were performed with Eu(II)EGTA1, Ti(III)citrate and sodium dithionite as electron donors, examining their effects on enzyme kinetics across different pH levels. Electrochemical data was used for comparison.
Eu(II)-based assays demonstrated a distinct pH dependence, with optimal activity at pH 5-6. Furthermore, the high reduction potential of Eu(II)EGTA (−0.88 V vs SHE) 1 relative to NaDT enabled the study of enzyme kinetics in solution assays across a broader range of solution potentials, facilitated by various redox mediators. A 200 mV change in solution potential resulted in a 35-fold increase in catalytic rate. Overall, the new Eu(II)-based assays generate solution data aligning more closely with protein film electrochemistry data, and result in CpI turnover frequencies greatly surpassing previous reports.
- (1) Vincent, K. A.; Tilley, G. J.; Quammie, N. C.; Streeter, I.; Burgess, B. K.; Cheesman, M. R.; Armstrong, F. A. Chemical Communications, 2003, (20), 2590.