Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Hydrogen Bond Donor or trans Donor: Spectroscopic Characterization and Reactivity Studies of Ferryl-oxo Intermediates Bearing a Pendant Alcohol Moiety (#575)
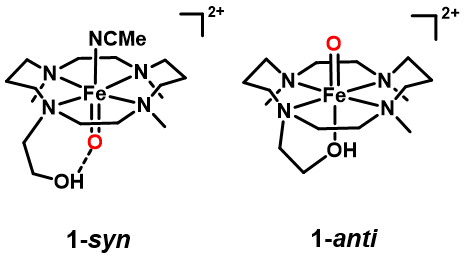
Increasing interest in the microenvironment1, 2 surrounding the active sites of different enzymes has recently led to many crucial structure-function-oriented discoveries3, 4. In light of this extensive research, high-valent ferryl-oxo species are frequently broached as the key reactive oxidants5-7. With the motivation to stabilize and understand the fundamental properties of such an iron(IV)-oxo center in trans tyrosine bound enzymes, catalases8, 9, vastly known for catalytic disproportionation reaction of hydrogen peroxide, we have investigated the effect of a pendant neutral alcohol moiety in the N-alkylated cyclam (1,4,8,11-tetraazacyclotetradecane) ligand backbone10. The oxo group binds to the site syn (1-syn) or anti (1-anti) (Figure 1) to the three methyl and –CH2CH2OH groups (TMC-HOR = 2-(4,8,11-trimethyl-1,4,8,11-tetraazacyclotetradecan1-yl)ethan-1-ol). Unlike in the previously reported [FeIV(Oanti)(TMC-SR)]+ (TMC-SR = 1-mercaptoethyl-4,8,11-trimethyl-1,4,8,11 tetraazacyclotetradecane) intermediate11, bearing an axial mono-anionic thiolate ligand trans to the oxo unit, the pendant alcohol moiety in 1-syn and 1-anti remains protonated as a prerequisite for the stabilization of the iron(IV)-oxo core in TMC-HOR ligand systems. It serves as a H-bonding donor to the iron(IV)-oxo unit in 1-syn, and stays bound trans to the iron(IV)-oxo moiety in 1-anti. Comparative reactivity study reveals 1-syn to be a stronger hydrogen atom abstraction and oxygen atom transfer agent relative to 1-anti. The different orientations of the oxo group, as well as the H-Bonding and trans effect, jointly contribute to the different spectroscopic and reactivity properties of 1-syn and 1-anti.
- Springer, B. A.; Sligar, S. G.; Olson, J. S.; Phillips, G. N., Jr., Mechanisms of Ligand Recognition in Myoglobin. Chemical Reviews 1994, 94 (3), 699-714.
- Wirstam, M.; Blomberg, M. R. A.; Siegbahn, P. E. M., Reaction Mechanism of Compound I Formation in Heme Peroxidases: A Density Functional Theory Study. Journal of the American Chemical Society 1999, 121 (43), 10178-10185.
- Krest, C. M.; Silakov, A.; Rittle, J.; Yosca, T. H.; Onderko, E. L.; Calixto, J. C.; Green, M. T., Significantly shorter Fe–S bond in cytochrome P450-I is consistent with greater reactivity relative to chloroperoxidase. Nature Chemistry 2015, 7 (9), 696-702.
- Yosca, T. H.; Rittle, J.; Krest, C. M.; Onderko, E. L.; Silakov, A.; Calixto, J. C.; Behan, R. K.; Green, M. T., Iron(IV)hydroxide pKa and the Role of Thiolate Ligation in C–H Bond Activation by CytochromeP450. Science 2013, 342 (6160), 825-829.
- MacBeth, C. E.; Golombek, A. P.; Young, V. G.; Yang, C.; Kuczera, K.; Hendrich, M. P.; Borovik, A. S., O2 Activation by Nonheme Iron Complexes: A Monomeric Fe(III)-Oxo Complex Derived From O2. Science 2000, 289 (5481), 938-941.
- Rohde, J.-U.; In, J.-H.; Lim, M. H.; Brennessel, W. W.; Bukowski, M. R.; Stubna, A.; Münck, E.; Nam, W.; Que, L., Crystallographic and Spectroscopic Characterization of a Nonheme Fe(IV)=O Complex. Science 2003, 299 (5609), 1037-1039.
- Ford, C. L.; Park, Y. J.; Matson, E. M.; Gordon, Z.; Fout, A. R., A bioinspired iron catalyst for nitrate and perchlorate reduction. Science 2016, 354 (6313), 741-743.
- Putnam, C. D.; Arvai, A. S.; Bourne, Y.; Tainer, J. A., Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism11Edited by R. Huber. Journal of Molecular Biology 2000, 296 (1), 295-309.
- Foroughi, L. M.; Kang, Y.-N.; Matzger, A. J., Sixty years from discovery to solution: crystal structure of bovine liver catalase form III. Acta Crystallographica Section D 2011, 67 (9), 756-762.
- Barman, D. J.; Lohmiller, T.; Katz, S.; Haumann, M.; Hildebrandt, P.; Nam, W.; Ray, K., An Oxoiron(IV) Complex Supported by an N-alkylated Cyclam Ligand System Containing a Pendant Alcohol Moiety. Chemistry – A European Journal 2025, n/a (n/a), e202404468
- Bukowski, M. R.; Koehntop, K. D.; Stubna, A.; Bominaar, E. L.; Halfen, J. A.; Münck, E.; Nam, W.; Que, L., A Thiolate-Ligated Nonheme Oxoiron(IV) Complex Relevant to Cytochrome P450. Science 2005, 310 (5750), 1000-1002.