Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Siderophore-like stealth substrates for chemoenzymatic synthesis of novel metal chelators. (#576)
Siderophores are secondary metabolites secreted by bacteria to assist in the uptake of iron from the environment. These natural products are optimised as Fe3+ chelators with stability constants exceeding 1030.1 One siderophore, desferrioxamine B (DFOB), has clinical relevance in its use as a treatment for acute iron poisoning and secondary iron-overload associated with haemoglobinopathies.2 New siderophore analogues with capacity to bind metal ions other than Fe3+ could be useful in a range of environmental and biomedical applications. One approach towards diversifying siderophore architectures is to exploit the native biosynthetic enzymes using chemoenzymatic synthesis. Here, the biosynthetic gene cluster DesABCD encoded by native-DFOB producing actinomycete species is being studied for this purpose,3 which orchestrates the biosynthesis of DFOB alongside other hydroxamic acid-containing siderophores.4 Of particular interest in this biosynthetic pathway is DesD, the final enzyme in this cluster which activates and condenses hydroxamic acid monomers of N-hydroxy-N-succinyl cadaverine (HSC) to form oligomeric species such as DFOG1 (3 HSC units) and DFOE, its macrocyclic counterpart.
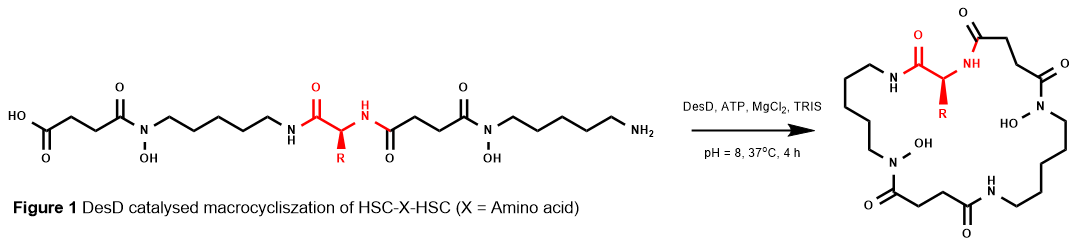
DesD has been shown to be able to accept non-native substrates for its condensation reactions5 and when provided HSC as the sole substrate, DesD condenses the HSC substrates first into DFOG1, then macrocyclises DFOG1 into DFOE, resulting in an almost quantitative conversion of HSC into DFOE. Exploiting these two properties, it is plausible that substitution of the internal unit of HSC in DFOG1 with a non-native substrate would still allow for macrocyclization by DesD (Figure 1). This could have the potential to generate new macrocycles by stealth with modified metal chelating properties. To this end, a selection of canonical amino acids were inserted within a linear HSC-X-HSC system, by employing solid-phase synthetic methods. Once synthesized, incubations with DesD produced a combinatorial pool of linear and macrocyclic products, amenable for screening upon metal ion addition to report new metal-siderophore chemistry.
- Zhang, G.; Amin, S. A.; Küpper, F. C.; Holt, P. D.; Carrano, C. J.; Butler, A., Ferric Stability Constants of Representative Marine Siderophores: Marinobactins, Aquachelins, and Petrobactin. Inorganic Chemistry 2009, 48 (23), 11466-11473.
- Codd, R.; Richardson-Sanchez, T.; Telfer, T. J.; Gotsbacher, M. P., Advances in the Chemical Biology of Desferrioxamine B. ACS Chemical Biology 2018, 13 (1), 11-25.
- Yang, J.; Banas, V. S.; Patel, K. D.; Rivera, G. S. M.; Mydy, L. S.; Gulick, A. M.; Wencewicz, T. A., An acyl-adenylate mimic reveals the structural basis for substrate recognition by the iterative siderophore synthetase DesD. Journal of Biological Chemistry 2022, 298 (8), 102166.
- Kadi, N.; Oves-Costales, D.; Barona-Gomez, F.; Challis, G. L., A new family of ATP-dependent oligomerization-macrocyclization biocatalysts. Nature Chemical Biology 2007, 3 (10), 652-656.
- K. P. Nolan, C. A. Rosser, J. L. Wood, J. Font, A. Sresutharsan, J. Wang, T. E. Markham, R. M. Ryan and R. Codd, An elastic siderophore synthetase and rubbery substrates assemble multimeric linear and macrocyclic hydroxamic acid metal chelators. Chemical Science, 2025, 16, 2180-2190.