Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Metal meets mucin: unraveling coordination, stability, and antimicrobial defense (#408)
The interplay between metal ions and antimicrobial peptides (AMPs) is key to designing next-generation bioinspired antimicrobial agents [1]. Human salivary AMPs are crucial components of the innate immune system, serving as a first line of defense against pathogens. These peptides, including those derived from the MUC7 protein, exhibit enhanced antimicrobial activity when complexed with metal ions [2]. Given their broad-spectrum activity and low propensity for resistance development, AMPs are considered promising candidates for novel therapeutic strategies. However, their clinical application is often limited by poor enzymatic stability, necessitating peptidomimetic modifications to improve both stability and antimicrobial efficacy [3].
In this study, we investigate the enzymatic stability, thermodynamics, coordination behavior, structural properties, and antimicrobial activity of Cu(II) and Zn(II) complexes with salivary proline-rich peptides and their D-amino acid-substituted analogs (Figure 1).
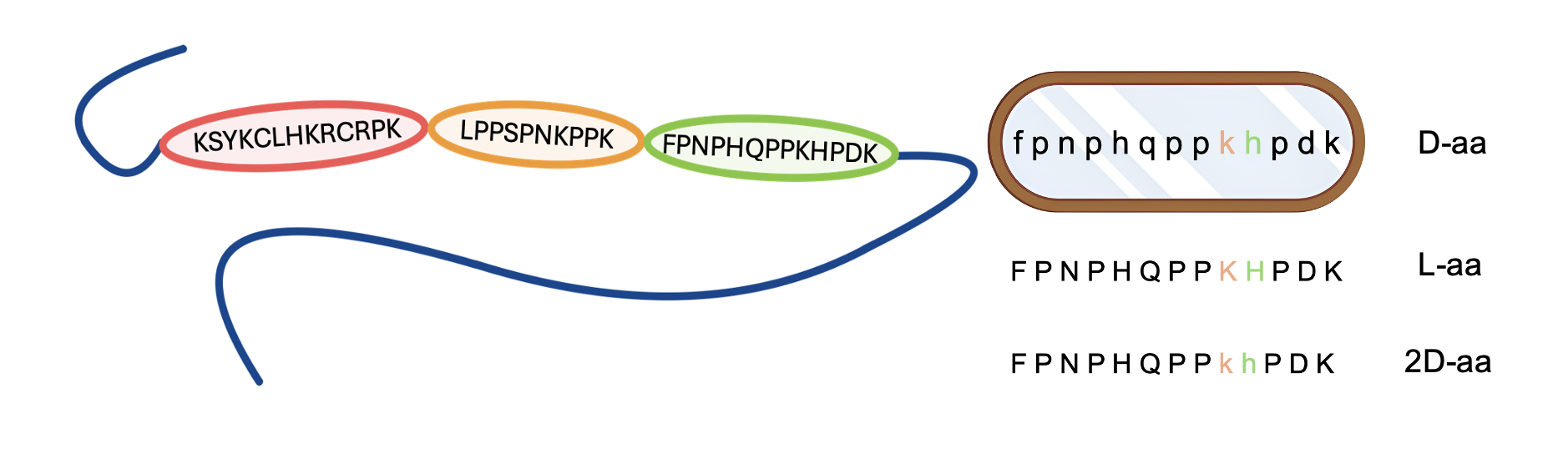
Figure 1. Simplified structure of mucin (left) with highlighted relevant fragments. Analyzed peptidomimetics of the FPN peptide (right), with enzymatically vulnerable regions marked in color. Lowercase letters in the sequence indicate D-amino acids.
A comprehensive analytical approach, integrating potentiometric titration, spectroscopic techniques (UV-Vis, CD, EPR), mass spectrometry, and HPLC, was employed to investigate the coordination behavior and stability of metal-peptide and peptidomimetic complexes. Biological assays further assessed their antimicrobial potential.
Our findings reveal that metal coordination preferences vary depending on the applied modifications. Enantiomeric substitution of amino acids significantly enhances the thermodynamic stability of Cu(II) and Zn(II) complexes, while the enzymatic stability of partially modified peptides remains unchanged. Notably, the fully D-amino acid analog exhibits exceptional resistance to proteolysis and, when complexed with metal ions, demonstrates the most favorable minimum inhibitory concentration (MIC) values, underscoring its potential for antimicrobial applications.
This study highlights the role of enantiomeric modifications in modulating metal-peptide interactions and optimizing stability, offering a promising strategy for designing next-generation antimicrobial peptides with enhanced therapeutic properties.
Acknowledgments
This work was financial supported by the Polish National Science Center under Grant No. UMO-2021/41/B/ST4/02654 (J.W.).
- [1] D. Łoboda, H. Kozłowski, M. Rowińska-Żyrek, New J. Chem., 2018,42, 7560.
- [2] K. Szarszoń, S. Andrä, T. Janek, J. Wątły, Inorg. Chem., 2024, 63, 11616.
- [3] J. Wątły, A. Miller, H. Kozłowski, M. Rowińska-Żyrek, J. Inorg. Biochem., 2021, 217, 111386.