Invited Talk 21st International Conference on Biological Inorganic Chemistry 2025
Routes to bioinspired nickel-carbonite entities and their interaction with metal ions (#43)
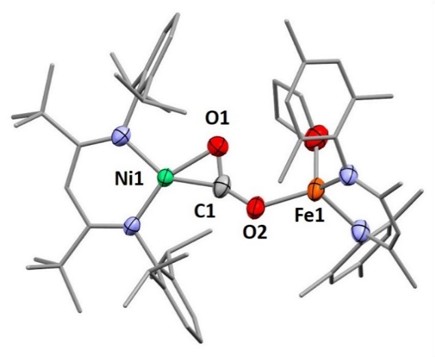
Reduced CO2 species are key intermediates in a variety of natural and synthetic processes. In the majority of systems, however, they elude isolation or characterisation due to high reactivity or limited accessibility and thus formulations often remain uncertain or based on calculations only. We report here on the generation of Ni–CO22-…M complexes (M = alkali, earth alkaline or transition metal ion) via hitherto rarely observed formate β‑deprotonation.1-4,6 The CO22- (carbonite) ligand is bound like a carbene to the nickel center and was found to interact with the counterions M+, introduced via the used bases or salt metathesis reactions. Through their properties they thus significantly influence the stability and reactivity of the complexes, which resemble the CO2 bound state in Ni,Fe carbon monoxide dehydrogenases (CODH).5 As in case of the latter, addition of a proton or other electrophiles leads to the elimination of CO. In particular the Ni–CO22-…Fe representative (Figure 1) resembles CO2-CODH intermediate in many ways.6 For the series of systems presented an inverse relation between redox behaviour and activation emerged: transition metal ions reduce the reduction potential of the carbonite unit but increase its tendency to undergo C-O bond cleavage.6
Figure 1:
- P. Zimmermann, S. Hoof, B. Braun-Cula, C. Herwig, C. Limberg, Angew. Chem. Int. Ed. 2018, 57, 7230-7233.
- P. Zimmermann, D. Ar, M. Rößler, P. Holze, B. Braun, C. Herwig, C. Limberg, Angew. Chem. Int. Ed. 2021, 60, 2312-2321.
- S. Wolff, V. Pelmenschikov, R. Müller, M. Ertegi, B. Cula, M. Kaupp, C. Limberg, Chem. Eur. J. 2024. DOI: 10.1002/chem.202303112
- S. Wolff, A. Ponsonby, A. Dallmann, C. Herwig, F. Beckmann, B. Cula, C. Limberg, Chem. Commun. 2024, 60, 5816-5819
- J. Fesseler, J.-H. Jeoung, H. Dobbek, Angew. Chem. Int. Ed. 2015, 54, 8560
- S. Wolff, A. M. Hofmann, K. B. Krause, K. Weisser, T. Lohmiller, C. Herwig, C. Limberg, Angew. Chem. Int. Ed. 2024. DOI: 10.1002/anie.202419675