Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Metal-responsive Up-regulation of Disulfide Compounds for Protein Folding Promotion under Stress Conditions (#404)
Metal ions are indispensable in many biological events, such as the stabilization of biomolecular assemblies and the mediation of signal transduction. However, even biologically essential metal ions can be cytotoxic when their homeostasis is imbalanced. Such metal stress severely hampers protein folding due to irreversible misfolding and aggregation, resulting in severe diseases.1 Although chelating compounds have been utilized for eliminating toxic metal ions, it has been overlooked how to restore native folding status under metal stress. Living cells are featured by stress response mechanisms in which folding-related enzymes are activated in response to oxidative stress and the resultant protein misfolding (Figure a). We believe that the stress-responsive up-regulation is a key to proteostasis under stress conditions. In this study, we developed a synthetic promotor of protein folding whose reactivity can be up-regulated under metal stress (Figure b).
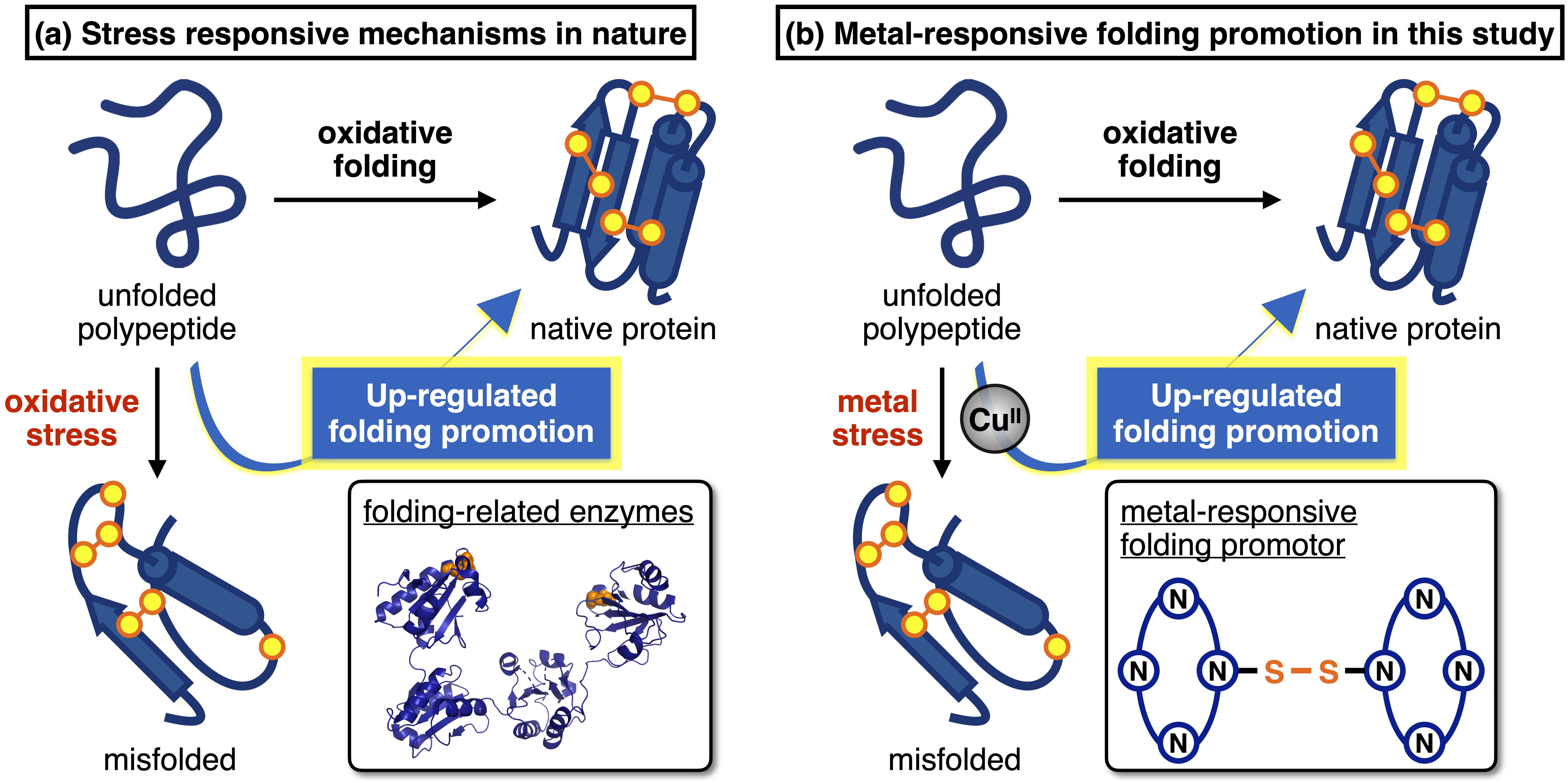
Synthetic disulfide compounds have been known as a functional oxidant which promotes protein folding accompanied by disulfide bond formation, namely oxidative protein folding.2 We developed a metal-responsive folding promotor, cyclam-SS, by conjugating two metal-binding cyclam ligands with a disulfide-containing linker. The oxidative strength of cyclam-SS was increased by the addition of CuII ions, possibly because cationic metal complexation destabilized the disulfide form and enhanced the reactivity. The performance of cyclam-SS as a folding promotor was examined using model substrate proteins. We found that oxidative protein folding by glutathione was severely hampered in the presence of CuII ions. On the other hand, the folding promotion by cyclam-SS was rather enhanced upon CuII addition, indicating stress-responsive up-regulation of folding promotion. Cyclam-SS was also applicable for promoting the oxidative folding of peptides which are pharmaceutically important but prone to aggregation. Furthermore, metal-dependent oligomerization of pathological proteins was suppressed by cyclam-SS, indicating its potential applicability to treatment of metal-related diseases.
Thus, we demonstrated metal-responsive promotion of oxidative protein folding by the synthetic disulfide compound.3 The concept of stress-responsive up-regulation would help design new pharmaceutical compounds and materials.
- Chen, L.; Min, J.; Wang, F. Signal Transduct. Target. Ther., 2022, 7, 378.
- Muraoka, T.; Okumura, M.; Saio, T. Chem. Sci., 2024, 15, 2282–2299.
- Mori, K.; Kuramochi, T.; Matsusaki, M.; Hashiguchi, Y.; Okumura, M.; Saio, T.; Furukawa, Y.; Arai, K.; Muraoka, T. under revision.