Poster Presentation 21st International Conference on Biological Inorganic Chemistry 2025
Bio-inspired nonheme iron oxygen activation (#402)
Nonheme iron enzymes are known to oxidize a range of substrates by catalyzing the activation of dioxygen. The Fe(IV)-oxido species are generally believed to be key reactive intermediates in nonheme iron enzymes for performing different transformations like halogenation, hydroxylation, olefin epoxidation etc. Spectroscopic studies implicate high-valent diiron species as intermediates in the oxidation chemistry of the diiron centers in methane monooxygenase (MMO) and ribonucleotide reductase (RNR), while mononuclear Fe(IV)-oxido units have been proposed as the oxidant for several mononuclear nonheme iron enzymes.1 The bispidine iron(IV)oxido species trans-N3-[(L)FeIV=O(Cl)]+ (S=1) is the most reactive nonheme iron model system known so far, with a reactivity similar to nonheme iron enzymes (C-H abstraction at cyclohexane, -80°C (propionitrile), t1/2 = 3.5 sec), and with 100% selectivity produces cyclohexyl chloride.2 In absence of organic substrates, there are various self-decay pathways, one leading to an oxido-bridged diiron(III) species. The reactivity of this “resting state” as well as reasons for the unprecedented reactivity of trans-N3-[(L)FeIV=O(Cl)]+ are discussed on the basis of spectroscopic study (see scheme 1).3
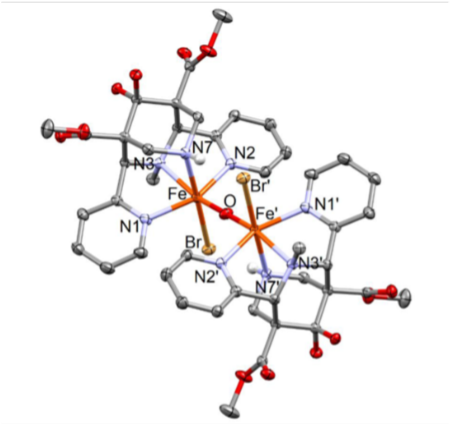
[L(Cl)FeIII-O-FeIII(Cl)L]2+
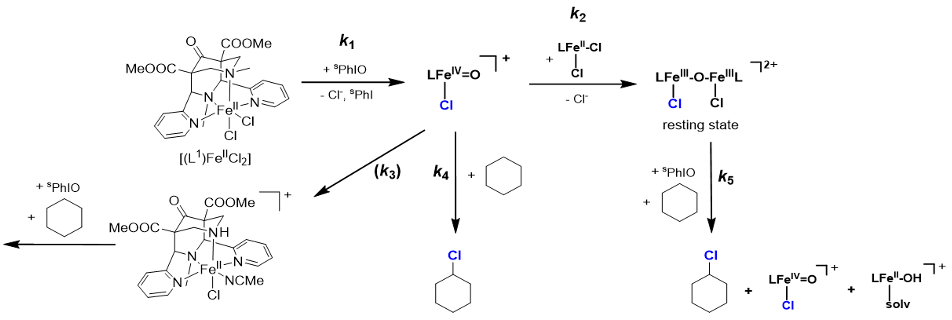
Scheme 1: Proposed mechanism of oxidation with high-valent iron-bispidine complexes.
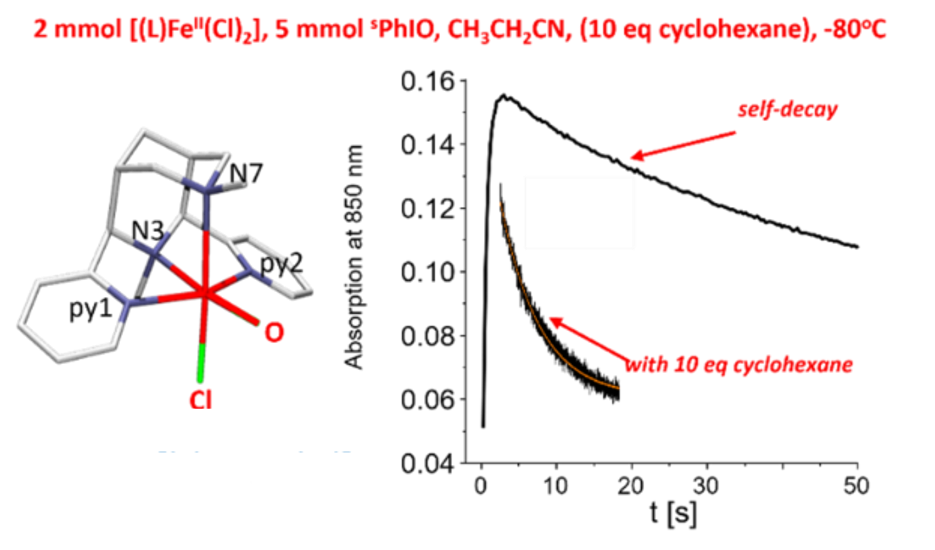
Figure 1: Time-dep. Formation and decay of [L(Cl)FeIV=O]+ and decay with cyclohexane.
- J. Hohenberger, K. Ray and K. Meyer, Nat. Commun., 2012, 3, 720.
- 2) Abu-Odeh, M.; Bleher, K.; Britto, N. J.; Comba, P.; Gast, M.; Jaccob, M.; Kerscher, M.; Krieg, S.; Kurth, M. Chem. Eur. J. 2021, 27, 11377-11390.
- 3) Bleher, K.; Comba, P.; Faltermeier, D; Gupta, A.; Kerscher, M.; Krieg, S.; Martin, B.; Velmurugan, G.; Yang, S. Chem. Eur. J., 2022 ,e202103452.